Through the 20th century, knowledge of the events occurring during cardiac development was clouded by conflicting descriptions, coupled with use of notably different terminologies. Furthermore, not all accounts were based on direct study of embryonic material, instead being constructed on the basis of interpretations of previous reports, supported by inferences made from the structure of the congenitally malformed heart. Such processes, in themselves, are understandable, since it is axiomatic that proper appreciation of the events occurring during formation of the heart will aid in the analysis of the morphogenesis of cardiac malformations, this being a desirable prerequisite in the search for optimal treatment.
Over the past decade, this has all changed. There has been an explosion of work, both anatomical and molecular, devoted to cardiac development. Advances in technology, coupled with the use of suitable animal models, now enable us to provide a more accurate account of the steps involved in formation and septation of the cardiac chambers. Not all of this new information is concordant with the “classical” accounts. In these reviews, therefore, we will describe, first, the steps involved in formation of the primary heart tube, and its conversion to the four cardiac chambers and the paired arterial trunks. We will then look in greater detail at the events occurring during the separation of the initial solitary heart tube into discrete systemic and pulmonary circulations.
FORMATION OF THE HEART TUBE
The mesodermal tissues that give rise to the heart first become evident when the embryo is undergoing the process known as gastrulation. In the human, this occurs during the third week of development, while for the mouse, at a comparable stage of development, around seven days will have elapsed from fertilisation, and the embryo will be in the presomitic stage. The embryonic plate in humans, initially possessing two layers, is ovoid, and is formed at the union between the yolk sac and the amniotic cavity. In the midline of the long axis of the oval disc is found the primitive streak, with the node at its cranial end. Through this streak, cells migrate from the upper layer by the process called gastrulation to form the three germ layers of the embryo proper: the ectoderm, the endoderm, and the mesoderm. The mesoderm insinuates between the ectodermal and endodermal layers, which themselves are continuous with the amnion and yolk sac, respectively. Having insinuated, the mesoderm spreads laterally and cranially within the embryonic disc, ultimately giving rise to a variety of structures, such as the somites, which will produce the axial structures, and the lateral plate mesoderm, which will form the parietal body wall. The cells that are destined to form the heart are also derived from this mesodermal layer. They form a crescent virtually at the cranial border of the disc (fig 1). As this heart forming region achieves its crescentic shape, the central region of the ectoderm transforms into the neural plate. This folds to become the neural tube, with the developing brain at its cranial end. In the human, the developing heart is initially cranial within the disc relative to the neural folds.
Figure 1.
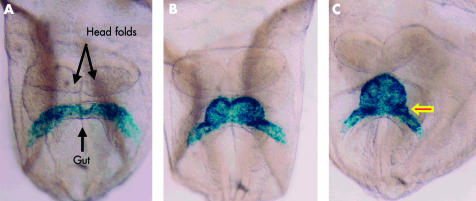
Ventral views of mouse embryos having four, six, and eight somites, respectively (panels A–C), representing the period from 7 ½12 to 8 days after fertilisation, in which the myocardium has been labelled blue using a reporter transgene for myosin light chain. The arrowed bulge in panel C is fated to contribute to the atrioventricular canal.
At this stage, the developing heart itself consists of a plate of promyocardial cells, intermingled with a plexus of endothelial strands, also derived from the cardiac crescent. The cardiac plate is positioned inferior to the presumptive pericardial cavity, which has arisen as a space within the mesoderm. With continuing folding of the disc, this heart forming region is moved into the developing neck of the embryo. The folding inverts the orientation of the developing heart relative to the neural structures and the gut. Initially the cardiac plate was inferior to the pericardial cavity but, subsequent to folding, it also folds into a tube between the pericardial space and the newly formed foregut that then becomes surrounded by the pericardial space. The process of folding is driven by the massive growth of the cranial end of the neural tube as it forms the brain, coupled with invagination of the endoderm to produce the foregut.
These events can now be visualised in animals such as the mouse, with the cardiac structures demonstrated by the genes and proteins they contain. The location of the promyocardial cells can be determined from the outset by the expression of NKX 2.5, a master gene controlling cardiac development.1 The cells can be then be shown by staining for sarcomeric proteins as they acquire a myocardial phenotype, revealing their location within the cardiac crescent (fig 1). The arrangement is somewhat different in mouse compared to human, since the murine embryonic plate is cup-shaped rather than discoid, with the endoderm on the outside and the ectoderm on the inside of the cup.
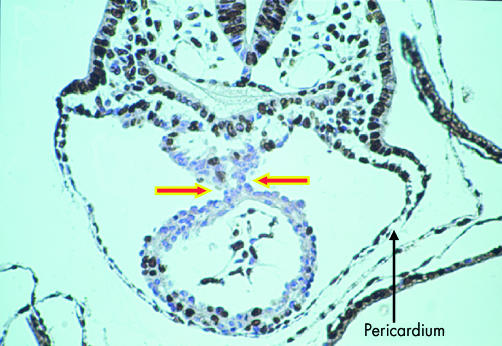
Irrespective of the differences between species, the endothelial plexus, as described above, is formed at the same time within the cardiac region and within the embryo, ensuring the presence of a circulatory system. In the cardiac region, this creates the primary endocardial tube of the heart. The endocardial cells forming the tube come from both sides of the developing embryo and, as they form a lumen, are enveloped by myocardial cells, all this occurring within the newly formed pericardial cavity. The myocardium at this stage, however, does not completely surround the endothelial tube. Instead, it retains, in its dorsal aspect, continuity with the splanchnic mesoderm of the developing mediastinum, through the structure known as the dorsal mesocardium (fig 2).
Figure 2.
This cross section is through the ventricular part of the heart tube of a mouse embryo with eight somites. At this stage, the tube is supported within the pericardial cavity by a mesocardium (arrows). The tissues have been incorporated with bromodeoxyuridine, which identifies dividing cells, labelled brown. The non-dividing cells are counterstained blue.
DEVELOPMENT OF THE HEART TUBE
At this stage, the forming heart is centrally positioned within the embryo, and is bilaterally symmetrical, taking the shape of an inverted Y (figs 1C and 3). The two arms of the Y, positioned inferiorly, are continuous with the developing venous tributaries of the embryo, yolk sac, and placenta. Marking studies, however, have shown that the arms of the initial primary tube are fated to become the precursors of the atrial chambers, with the stem of the Y itself becoming the definitive left ventricle.2 There is no evidence to support the notion that, from the outset, the “straight heart tube” contains all components of the definitive cardiac chambers.3 It is only as the symmetrical arms of the tube are incorporated caudally into the heart to form the primary atrial component that there is formation also of a prominent junctional component, the atrioventricular canal. Subsequent to these changes, the venous tributaries then drain to either side of the newly formed atrium through the right and left sinus horns. This occurs in symmetrical fashion in the mouse but, when first seen in the human, the venous tributaries are already asymmetrical.4 At the stage when the systemic venous tributaries are already seen draining to the newly incorporated primary atrium, there is no formation of either the lungs or the pulmonary vein. Establishment of the pulmonary circulation, and its connection with the heart, is a later event in development5.
Figure 3.
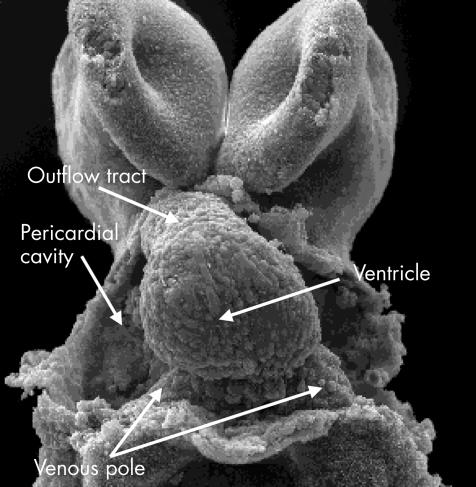
This scanning electron micrograph, viewed from the front in a mouse with 11 somites, shows the stage of the “straight heart tube”. Note that the tube is composed primarily of the ventricle, which will become the definitive left ventricle. As yet, there is no formation of an atrial component, and the outlet is similarly rudimentary.
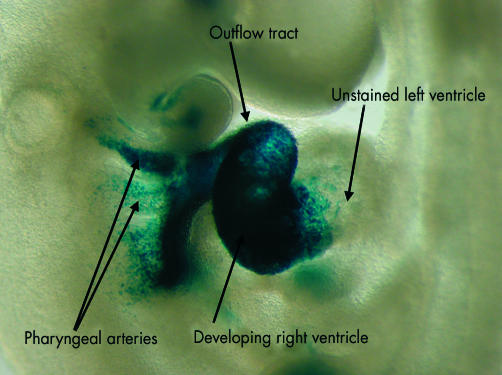
By this time, nonetheless, other important changes have been occurring at the cranial end of the heart tube. Cells from a second cardiogenic area, located posterior to the dorsal wall of the developing pericardial cavity (fig 4), migrate into the cardiac region, where they make significant contributions to the developing cranial pole of the heart tube. The cells from this secondary heart field,6–8 first identified as a second crescent within the embryonic disc, lying contiguous with, but medial to, the primary cardiac crescent, will populate the outflow tract and the primordium of the right ventricle (fig 5).
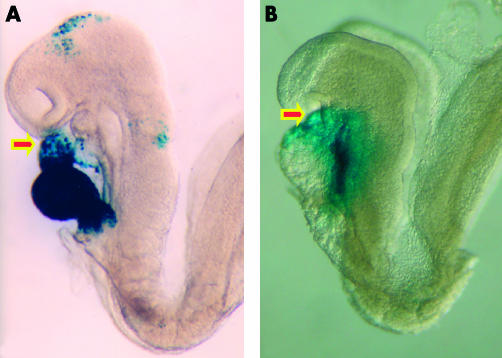
Figure 4.
These embryos are photographed in left lateral view. The embryo shown in panel A carries a transgenic marker for myosin light chain, and shows staining throughout the derivatives of the primary heart field. Note that the outflow region (arrowed) is only partly stained. The embryo shown in panel B carries a transgenic marker for fibroblast growth factor 10. This marks the initial site of the contributions made to the developing heart from the secondary heart field, staining the outflow tract (arrowed) and developing right ventricle (see also fig 5).
Figure 5.
In this embryo, the cells from the secondary heart field, shown in fig 4B, are labelled with a reporter transgene for fibroblast growth factor 10. As can be seen, the cells from the secondary field populate the pharyngeal arches, the outflow tract, and the developing right ventricle. They do not, however, extend beyond the interventricular groove to enter the left ventricle.
LOOPING OF THE HEART TUBE
These caudal and cranial additions to the tube produce a pronounced elongation of the primary heart tube. Associated with this elongation, the dorsal mesocardium, initially tethering the developing left ventricle to the mediastinum, undergoes disruption and liberates the larger part of the tube. Once liberated, the tube itself bends to the right as the start of the process known as looping (fig 6). Looping of the heart tube is usually held to be the first visual evidence of asymmetry within the embryo, although the atrioventricular canal is itself formed in asymmetric fashion, with a bulge to the left (arrow in fig 1C). The signalling pathway that ensured that the loop bent to the right, however, is established earlier, during the stage of gastrulation. At this earlier stage, a leftward flow of secreted proteins across the node is the start of at least two separable pathways.9 One of these, of which we currently know very little, ensures that the loop turns in rightward direction. The other ensures that some organs in the body, including parts of the heart, develop with morphologically left sided or right sided features. The pathways involve genes including lefty, nodal, and Pitx2.10
Figure 6.
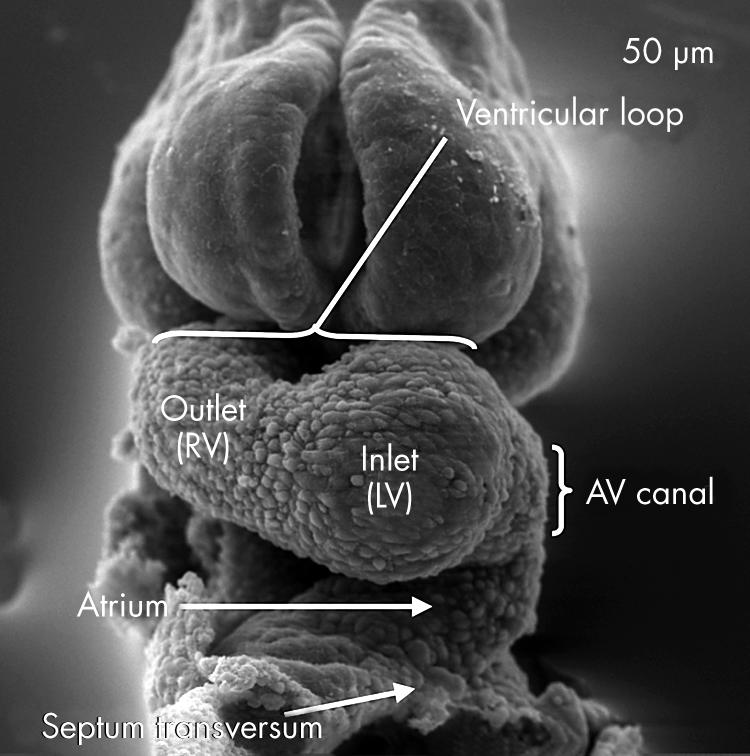
In this scanning electron micrograph, from a mouse embryo with 12 somites, the ventricular component of the heart tube has looped to the right. It now possesses two segments, which develop in sequence. The apical part of the left ventricle (LV) will balloon out from the inlet part of the loop, while the apical part of the right ventricle (RV) will grow from the outlet part. Note also that there is now an atrioventricular canal formed between the ventricular loop and the developing atrial segment.
Once formed, the ventricular loop itself has inlet and outlet components, with the outlet part supporting the outflow tract. The outflow tract, in turn, feeds the arteries that arise from the aortic sac and extend into the increasing number of pharyngeal arches.11 This arrangement is seen at around the 25th day in the human, the comparable situation being the 11th day in the mouse, when there has been formation of about 40 somites. The stage is now set for formation of the definitive cardiac chambers, along with the arterial trunks.
FORMATION OF THE CARDIAC CHAMBERS
By the stage of looping, the primary heart tube within the pericardial cavity can be divided into atrial and ventricular components along with an outflow tract. The atrial and ventricular components are separated by the atrioventricular canal, which at this stage has significant length (fig 7). The systemic venous tributaries, embedded in the substance of the posterior mediastinum, drain into the unseptated primary atrium. The blood flowing through the atrial component of the tube must, perforce, traverse the entirety of the ventricular loop so as to reach the outflow tract. This developing outflow component is supported by the distal, or outlet, component of the ventricular loop. By this stage, a constriction, which marks the site of the primary interventricular foramen, has developed between the inlet and outlet parts of the ventricular loop, which will become the left and right ventricles, respectively. The myocardial walls of the heart tube at this stage are formed of so-called primary myocardium.12 With further development, pronounced changes occur in all parts of the tube so as to produce separate left and right components. These changes occur over the same period of time but, for convenience, we will describe them sequentially.
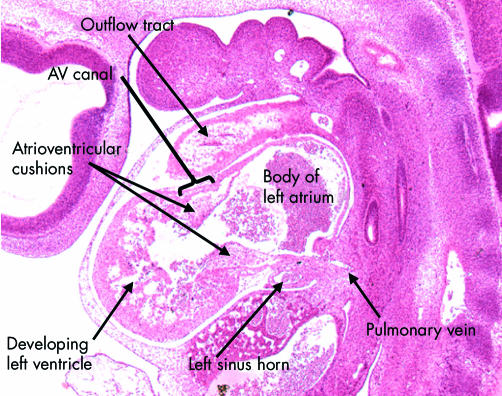
Figure 7.
This sagittal section is from a human embryo at Carnegie stage 14. It shows the developing left ventricle in communication with the atrial component of the primary heart tube through the atrioventricular canal, which at this stage has considerable length. Note the presence of the atrioventricular endocardial cushions on the luminal aspect of the canal. Note also the location of the pulmonary vein, which at this stage opens inferiorly to the primary atrial component.
FORMATION OF THE ATRIUMS
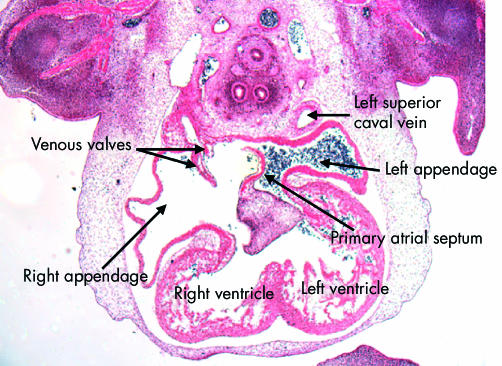
Completion of the development of the left atrium requires formation of the lungs and the pulmonary vasculature. The lungs themselves develop as outpouchings from the trachea. As the lung buds form, a plexus of vessels develops around them. The plexus establishes a connection with the primary atrial component of the heart tube via the pulmonary vein. Initially seen as an endothelial strand within the mediastinum, the initially solitary vein makes contact with the heart through the dorsal mesocardium (fig 8). By this time, the asymmetry of the systemic venous tributaries has become exaggerated, with the left sinus horn, cradling the developing pulmonary vein, becoming incorporated in the left side of the atrioventricular junction but opening into the right side of the primary atrium. The left horn always maintains its own walls within the left atrioventricular junction, separate from those of the primary atrium. When viewed internally, the junction between the systemic venous tributaries and the primary atrium can now be recognised because of the formation of the venous valves. The opening of the solitary pulmonary vein is now seen in the left and inferior part of the primary atrium, positioned between the folds that mark the site of the persisting dorsal mesocardium (fig 9). Concomitant with these changes, the superolateral walls of the atrial component of the primary heart tube have ballooned out to either side of the outflow tract to form the atrial appendages. It is formation of the two appendages that first differentiates the morphologically right and left sides of the primary atrium. This morphological sidedness is under the control of the pathways discussed above, mediated finally by Pitx2.10 The right appendage is extensive. As the systemic venous sinus is incorporated within the forming right atrium, it insinuates itself between the appendage and the developing primary septum. The appendage is continuous distally with the expanding vestibule of the right atrium, formed by incorporation of the musculature of the atrioventricular canal into the developing atriums.13 Within the left atrium, a much larger contribution to the definitive chamber is made by the atrial component of the primary tube, from which the left appendage arises as a narrow outpouching positioned superiorly and to the left (fig 10). As with the developing right atrium, part of the initial atrioventricular canal becomes incorporated into the definitive left atrium as the vestibule of the mitral valve.13 The pulmonary venous component, when first formed in the human, is relatively insignificant, since a solitary vein opens inferiorly (fig 7). Only subsequent to septation does the venous component expand to form the roof of the left atrium, eventually producing the definitive arrangement with four venous orifices.6
Figure 8.
This section, cut in the frontal plane, in a human embryo at Carnegie stage 16, shows the entry of the pulmonary vein to the inferior aspect of the left side of the primary atrial component. Note that the vein has entered through the dorsal mesocardium, and note its relation to the left sinus horn, which will persist as the coronary sinus.
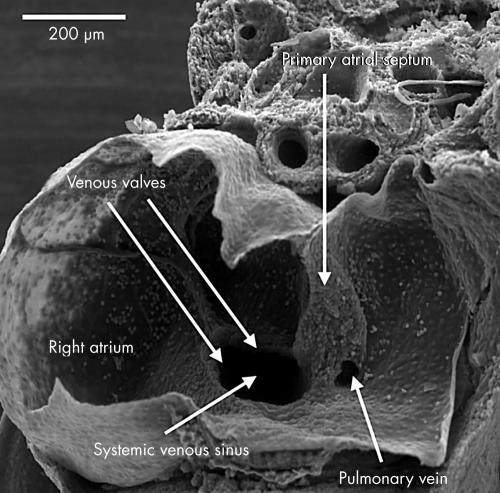
Figure 9.
This scanning electron micrograph shows a dissection of the atrial chambers in a mouse embryo with 43 somites, in the second half of the 11th day of development. The systemic venous sinus is now enclosed by the venous valves, and is incorporated within the right side of the primary atrial component. The pulmonary vein opens as a solitary channel to the inferior part of the left side of the primary atrium, entering between the ridges representing the remnants of the dorsal mesocardium. Note the primary atrial septum, which is growing down from the roof of the primary atrium.
Figure 10.
This section, in the frontal plane, is from the human embryo at Carnegie stage 16. It shows the difference, from the outset, in the arrangement of the atrial appendages. The left appendage arises superiorly from a narrow neck in the left side of the primary atrial component. The right appendage, in contrast, is much broader, and is separated by the systemic venous sinus, enclosed within the venous valves, from the atrial septum. Note that the primary septum has now grown down and fused with the superior atrioventricular endocardial cushion. Note also that the left superior caval vein possesses its own walls outside the developing left atrium.
FORMATION OF THE VENTRICLES
The ventricles are derived from the ventricular loop. Initially, the ventricular part of the primary tube was formed by the stem of the Y shaped heart tube, derived from the primary cardiac crescent, and by a distal part that received significant contributions from the secondary heart field. At this stage, all the blood from the atrial segment was required to pass through these two parts of the primary tube so as to reach the outflow tract. As the tube bent, the primary interventricular foramen became obvious between its two components. After looping, the tube itself has an inner and an outer curvature (fig 11). Pronounced changes occur in both of these curves. The apical parts of the two ventricles balloon from the outer curve, with the inlet part of the primary tube giving rise to the developing apical part of the left ventricle, and the outlet part being the origin of the developing apical component of the right ventricle.14–16 The beginnings of formation of the apical part of the left ventricle are seen even before looping. Once formed, the new myocardium can readily be distinguished from the primary myocardium by its expression of atrial natriuretic factor.12 The apical part of the right ventricle appears subsequently, ballooning from the outlet limb of the primary tube (fig 12). It is the trabeculations of these outpouchings that eventually give the definitive ventricles their characteristic morphology. Unlike the atrial chambers, the morphological differences between the two ventricles are not a reflection of left–right asymmetry. It is more likely that they reflect the spatio-temporal development of the ventricles in series within the ventricular component of the primary heart tube.
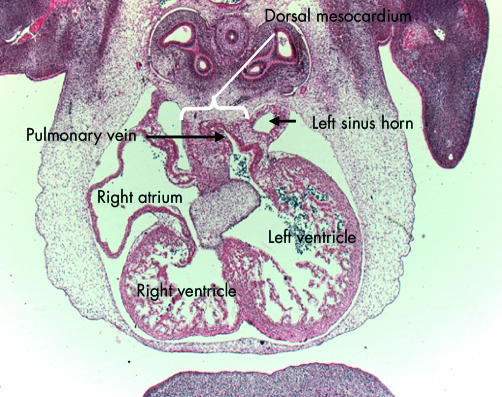
Figure 11.
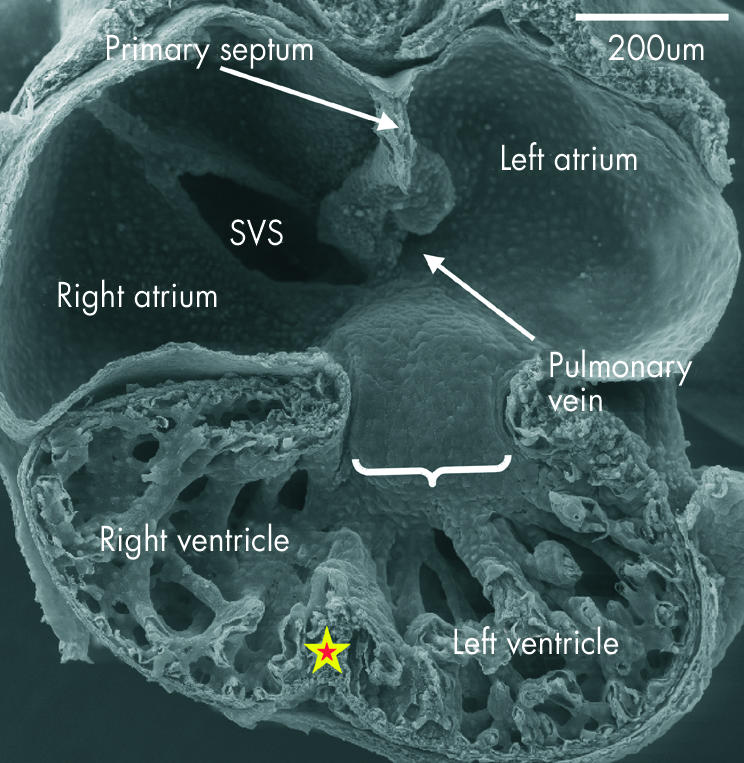
This scanning electron micrograph shows the posterior half of a mouse embryo with 40 somites transacted in the frontal plane. The systemic venous sinus (SVS) is incorporated within the right side of the primary atrium, and the ridges marking the site of the dorsal mesocardium are seen inferiorly in the left side. The pulmonary vein opens between these ridges (see fig 9). The atrioventricular canal at this stage, shown by the bracket, with the inferior cushion occupying most of its wall, is connected through the larger part of its circumference with the inlet part of the ventricular component of the primary heart tube, from which is ballooning the apical part of the developing left ventricle. Note the small size, at this stage, of the developing right and left atrioventricular orifices. The apical part of the developing right ventricle is ballooning from the outlet part of the primary tube, and the primordium of the muscular ventricular septum, shown by the star, is seen between the two pouches.
Figure 12.
These sections, from human embryos, show the fate of the primary interventricular foramen. This is shown in the upper panel, from an embryo at Carnegie stage 15, in which the larger circumference of the atrioventricular canal is supported by the inlet component of the ventricular loop, from which has ballooned the apical part of the left ventricle. The muscle surrounding the interventricular foramen (shown by the double headed arrow) is demarcated by an antibody to the nodose ganglion of the chick. The lower panel shows the stage subsequent to expansion of the atrioventricular canal, representing Carnegie stage 16 or 17. The right side of the atrioventricular canal itself has now been incorporated into the right atrium as the vestibule of the forming tricuspid valve. The location of the tissue staining positive for the antibody (single red arrow) shows that the inlet of the right ventricle has been derived from the outlet component of the ventricular part of the primary heart tube. Note that the tissue demarcating the location of the initial interventricular foramen retains its location on the crest of the muscular septum (between green arrows), but is also extending behind the outflow tract (double red arrow) concomitant with the transfer of the subaortic outlet to the developing left ventricle.
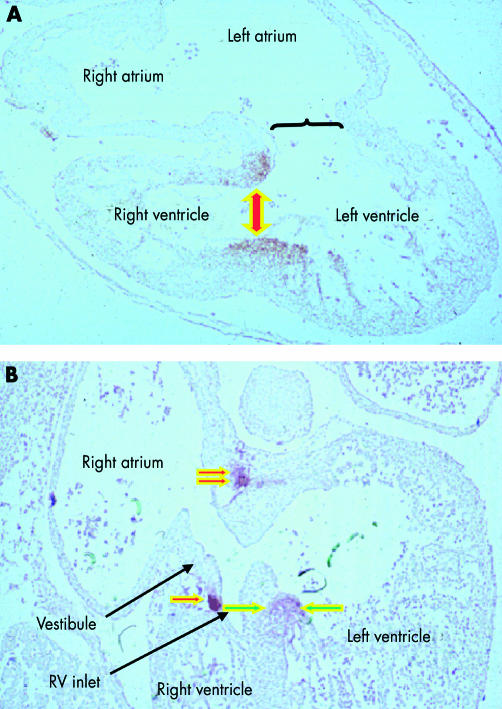
The changes that occur within the inner curve ensure that each apical part achieves its own inlet and outlet component. When ballooning of the apical components commences, the walls of the atrioventricular canal are joined almost exclusively to the developing left ventricle, while the outlet component of the heart tube is supported almost entirely by the developing right ventricle (fig 11). At this stage, the myocardium surrounding the interventricular foramen itself, the so-called primary ring (fig 13), can be distinguished within the primary myocardium by its expression of the GlN epitope.17
Figure 13.
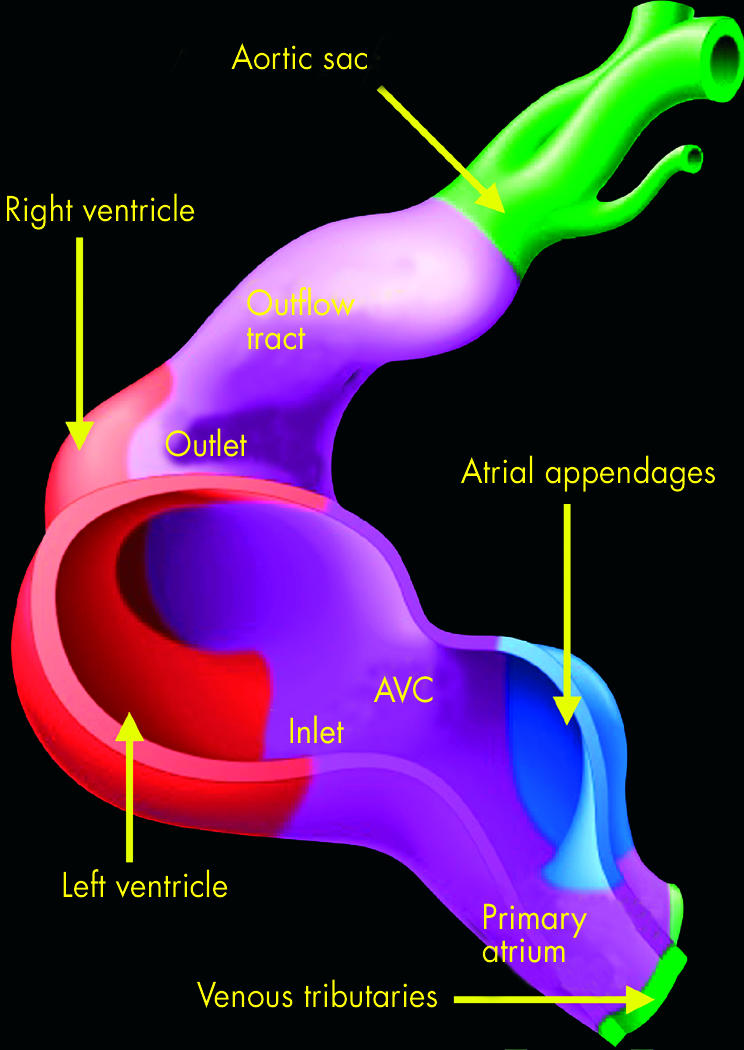
This illustration shows the origin of the components of the developing atriums and ventricles. The myocardium of the primary heart tube is shown in purple, and makes up the primary atrium, the atrioventricular canal (AVC), the inlet and outlet components of the ventricular loop, and the outflow tract. Shown in green are the systemic venous tributaries, which are eventually incorporated within the right atrium, and the aortic sac with its arterial branches. The pulmonary vein is not shown, this being a new development appearing concomitant with the formation of the lungs. The atrial appendages, shown in blue, balloon in parallel from the primary atrial component of the heart tube. The apical parts of the ventricles, in contrast, balloon in series from the primary tube, with the apical part of the left ventricle growing from the inlet component, and the apical part of the right ventricle from the outlet component.
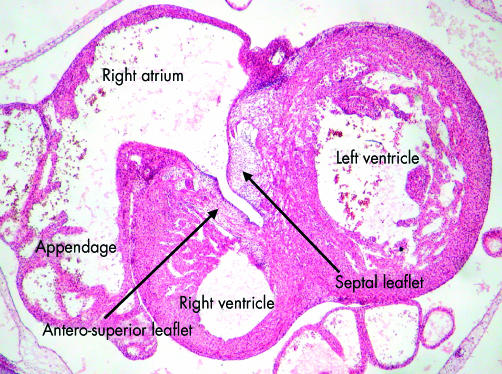
Tracing this primary ring over a period of time demonstrated the remodelling of the ventricular segment of the inner heart curvature, along with its junction with the atrioventricular canal proximally and the outlet component distally. This remodelling permits the separating atriums, and the dividing outflow tract, to be shared between the developing apical components of the left and right ventricles. Sharing of the atriums between the ventricles requires expansion of the atrioventricular canal. From the outset, the wall of the developing right atrium is continuous in the inner curvature, via the primary fold, with the wall of the outflow tract. All that is required for the cavity of the right atrium to achieve direct continuity with that of the developing right ventricle, therefore, is expansion of the atrioventricular canal. Subsequent to expansion, and concomitant with the development of the insulating plane between the atrial and ventricular chambers, the musculature of the right side of the atrioventricular canal itself then becomes incorporated into the right atrium as the vestibule of the tricuspid valve.13 The inlet of the right ventricle, contiguous with the vestibule of the tricuspid valve, develops within the ventricular component of the primary heart tube, specifically in the part delineated as the primary ring by its expression of GlN. The leaflets of the tricuspid valve delaminate from the myocardial walls of the primary tube, incorporating the endocardial cushions in their substance (fig 14). The cavity of the left atrium is continuous with that of the developing left ventricle from the outset. As with the right side, the musculature of the atrioventricular canal becomes sequestrated within the atrium subsequent to formation of the atrioventricular plane of insulation, the leaflets of the mitral valve delaminating within the inlet component of the left ventricle in a fashion comparable to the formation of the tricuspid valve (fig 14). Completion of left ventricular development requires that half of the proximal part of the initial outflow tract be transferred from its initial location above the developing right ventricle to form the aortic vestibule, leaving the remainder of the outflow tract as the subpulmonary infundibulum. This change, obviously, requires that significant changes occur also within the outflow tract itself.
Figure 14.
This section, from a human embryo at Carnegie stage 20, just subsequent to the completion of ventricular septation, shows how the leaflets of the tricuspid valve are delaminating within the part of the primary heart tube from which ballooned the apical component of the right ventricle. As the leaflets delaminate from the superficial layers of ventricular myocardium, they incorporate the endocardial cushions within their substance, subsequently being transformed into fibrous tissue.
FORMATION OF THE ARTERIAL TRUNKS
The outlet component of the primary heart tube, extending from the distal part of the ventricular loop to the distal extent of the pericardial cavity where it joins the aortic sac, is initially a structure with exclusively myocardial walls, and with distal and proximal parts separated by a characteristic bend (fig 15). Due to processes as yet undetermined, the walls of the distal outflow change rapidly from this myocardial phenotype (fig 16) to an arterial one (fig 17). Concomitant with the changes, the initially solitary tube seen distally is replaced by the intrapericardial portions of the ascending aorta and the pulmonary trunk. The proximal part also separates into two components, again losing its myocardial phenotype, with the arterial valvar leaflets and their supporting arterial sinuses formed just proximal to the bend, which marks the site of formation of the definitive sinutubular junctions (fig 18). The most proximal part of the outflow tract is then itself separated by fusion of the cushions within it, new myocardium forming within the cushions to produce the medial part of the subpulmonary infundibulum, which retains its origin from the right ventricle.18 At the same time, the subaortic part of the outflow segment is partitioned to the left ventricle by the fusion of the cushions to the crest of the muscular ventricular septum, the myocardium of the initial inner heart curvature (fig 16) eventually disappearing to permit fibrous continuity between the leaflets of the aortic and mitral valves in the ventricular roof.
Figure 15.
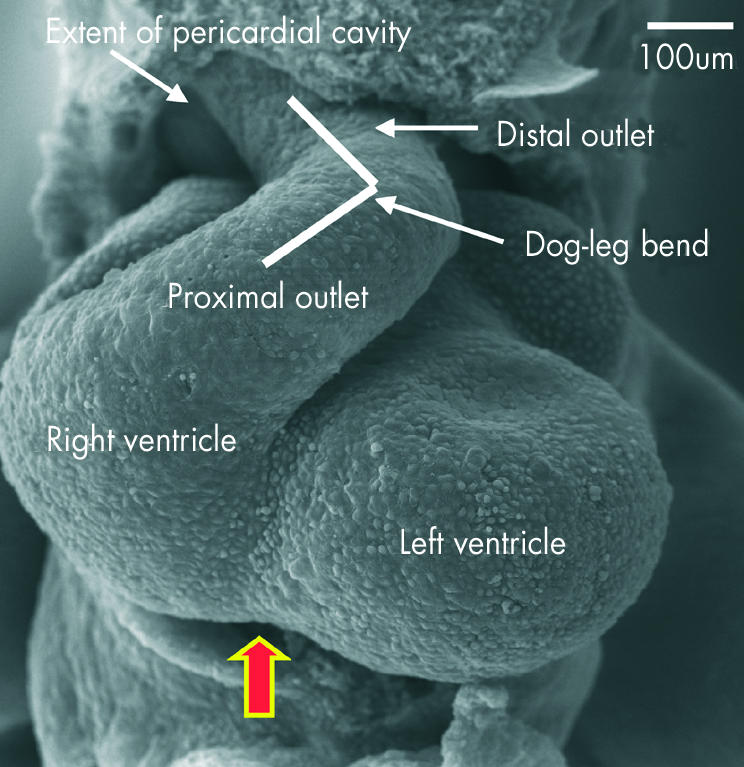
This scanning electron micrograph, from a mouse with 40 somites, shows how the outflow tract, now supported by the developing right ventricle, has bent into two discrete components separated by a dog-leg bend. The distal part extends to the border of the pericardial cavity, where it becomes continuous with the aortic sac. The arrow shows the interventricular groove.
Figure 16.
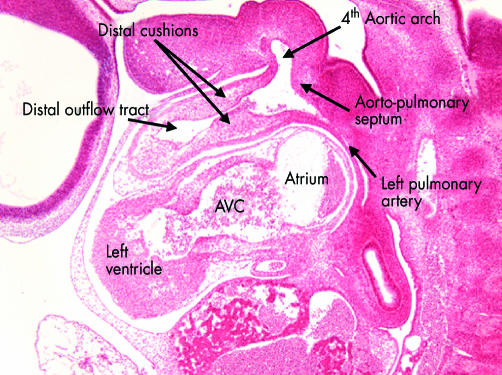
This sagittal section is from the human embryo at Carnegie stage 14 also illustrated in fig 7. This section is taken through the distal part of the outflow tract, and shows the distal cushions that will divide this part into the intrapericardial portions of the aorta and pulmonary trunk (see figs 17 and 18). Note the location of the aortopulmonary septum within the aortic sac, separating the origins of the fourth and sixth arches. In this section the left pulmonary artery is seen arising from the artery of the sixth arch. AVC, atrioventricular canal.
Figure 17.
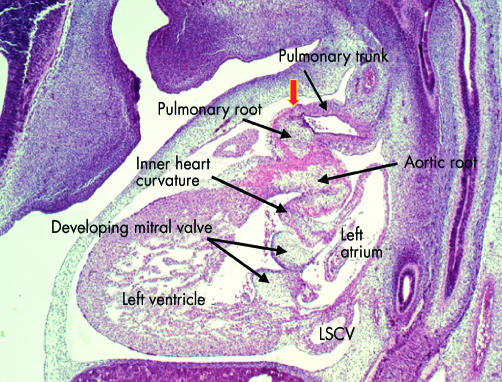
This sagittal section is from a human embryo at Carnegie stage 18, just before the completion of cardiac septation. Note that the distal outflow tract has now separated into the intrapericardial components of the pulmonary trunk, which is sectioned, and the aorta, which cannot be seen in this section. The arterial roots at this stage, however, are still encased within the muscular wall of the proximal outflow tract. The sinutubular junction, shown by the arrow, is formed at the site of the initial dog-leg bend (see fig 15). Note the cushions fusing to form the aortic leaflet of the mitral valve, which at this stage is still separated by the musculature of the inner heart curvature from the developing leaflets of the aortic valve. Note also the left superior caval vein (LSCV) now incorporated within its own wall within the left atrioventricular groove.
Figure 18.
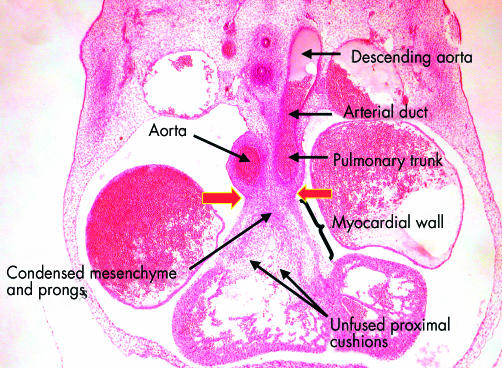
This frontal section, from a human embryo at Carnegie stage 16, shows the distinction between the proximal and distal parts of the outflow tract. The distal part has been separated by the distal cushions into the intrapericardial portions of the aorta and the pulmonary trunk, the walls of which now possess an arterial phenotype. Note the continuation of the pulmonary trunk through the arterial duct, derived from the sixth arch, to the descending aorta. The proximal outflow tract, in contrast, is still enclosed within muscular walls. The arrows show the site of the developing sinutubular junction, formed at the initial dog-leg bend. Note that the proximal cushions are fused distally, with the site of fusion marked by condensed mesenchyme. The more proximal parts of the cushions, however, penetrated by prongs from the condensed mesenchymal mass, remain unfused. Completion of fusion, followed by muscularisation, will convert this area into the supraventricular crest of the right ventricle.
CONCLUSIONS
The major step in formation of the separate atrial and ventricular chambers is the ballooning of secondary myocardium from the outer curvature of the primary heart tube. The outpouchings that give rise to the appendages of the atrial chambers are derived initially from the same segment of the primary tube. The differentiation of these structures into morphologically right or left components is under the control of genes such as Pitx2, which determine left–right asymmetry.10 The venous components of the atrial chambers, which represent the atrial inlets, appear sequentially. The systemic venous tributaries are in continuity with the primary atrial component from the outset of development, being part of the original circulatory system of the embryo. With remodelling, the entirety of this “sinus venosus” is shifted to open into the right side of the primary atrium. The pulmonary venous component of the left atrium, in contrast, is a new development. It appears concomitant with formation and vascularisation of the lung buds, using the remnant of the dorsal mesocardium at the venous pole to gain its access to the left side of the primary atrium, which persists as the extensive body of the left atrium. The initial atrial outlet, the atrioventricular canal, becomes incorporated into both definitive atriums as the vestibules of the atrioventricular valves.13 Eventual separation of the atrial from the ventricular myocardium by formation of fibro-adipose tissue at the atrioventricular junctions is a late event, occurring subsequent to the completion of septation.19
The basis of formation of the ventricles is the outpouching of their apical components from the inlet and outlet parts of the ventricular component of the primary heart tube, respectively.14–16 It is the development of characteristic trabeculations within these apical parts that gives the definitive ventricles their morphologically right and left identity. This process is not under the control of Pitx2 and its associated genes since, while the atrial appendages develop in parallel from the primary atrial component of the tube, the apical ventricular components develop in series within the ventricular part of the heart tube. Thus, isomerism can be seen in the atrial, but not the ventricular components of the developing heart. The inlets and outlets to each definitive ventricle are produced by remodelling of the inner curvature of the primary heart tube, this then setting the scene for the completion of septation. The outlet component of the primary heart tube, initially with myocardial walls, is replaced distally by the intrapericardial components of the aorta and the pulmonary trunk, with the processes involved in this separation still to be established. The proximal part of the outflow tract becomes converted, and separated, into the arterial valves, their supporting arterial sinuses, and the subarterial ventricular outflow tracts. It is the complicated processes involved in the completion of septation within the atrial, atrioventricular, ventricular, and arterial regions that will be the focus of our next reviews.
REFERENCES
- 1.Lints TJ, Parsons LM, Hartley L, et al. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 1993;119:419–31. [DOI] [PubMed] [Google Scholar]
- 2.de la Cruz MV, Sanchez-Gómez C, Cayre R. The developmental components of ventricles: their significance in congenital malformations. Cardiol Young 1991;1:123–128. ▸ Maria Victoria de la Cruz was one of the first embryologists to recognise the fact that all parts of the developing heart were not already present at the “straight tube” stage. This paper is a review of her earlier works, and puts the embryological studies into the context of the morphology of congenital cardiac malformations. [Google Scholar]
- 3.Black BL, Olson EN. Control of cardiac development by the MEF2 family of transcription factors. In: Harvey RP, Rosenthal N, eds. Heart development. London: Academic Press, 1999:131–42. ▸ A good chapter in an excellent textbook. As with many of the other chapters, however, the value of the observations concerning advances in molecular biology are devalued to some extent by slavish adherence to concepts of “armchair embryology”.
- 4.Knauth A, McCarthy KP, Webb S, et al. Interatrial communication through the mouth of the coronary sinus defect. Cardiol Young 2002;12:364–72. ▸ An important study showing that, from the start of its development, the coronary sinus, derived from the left sinus horn, possessed its own walls discrete from those of the left atrium. [DOI] [PubMed] [Google Scholar]
- 5.Webb S, Kanani M, Anderson RH, et al. Development of the human pulmonary vein and its incorporation in the morphologically left atrium. Cardiol Young 2001;11:632–42. ▸ Conventional wisdom has suggested that the pulmonary vein is derived from the embryonic systemic venous sinus, or “sinus venosus”. This study showed that there is no evidence to support this notion, since the incorporation of the pulmonary vein to the left atrium is a late event in cardiac development. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 2001;1:435–40. [DOI] [PubMed] [Google Scholar]
- 7.Waldo KL, Kumiski DH, Wallis KT, et al. Conotruncal myocardium arises from a secondary heart field. Development 2001;128:3179–88. [DOI] [PubMed] [Google Scholar]
- 8.Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, et al. The outflow tract of the heart is recruited from a novel heart-forming field. Devel Biol 2001;238:97–109. ▸ References6 through8 are the original studies that revealed the presence of a second heart forming field, discrete from the primary cardiac crescent. The secondary field gives rise to the outflow tract, and probably to the greater part of the developing right ventricle. [DOI] [PubMed] [Google Scholar]
- 9.Nonaka S, Shiratori H, Saijoh Y, et al. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 2002;418:96–9. [DOI] [PubMed] [Google Scholar]
- 10.Harvey RP. Patterning the vertebrate heart. Nat Rev Genet 2002;3:544–56. ▸ This excellent review, by one of the editors of the book cited in reference4, redresses the balance between molecular biology and morphology, setting the exciting new findings of the past decade into an appropriate understanding of morphological events. [DOI] [PubMed] [Google Scholar]
- 11.Hiruma T, Nakajima Y, Nakamura H. Development of pharyngeal arch arteries in early mouse embryo. J Anat 2002;201:15–29. ▸ In this exquisite study, a group from Japan have re-investigated the formation of the developing system of aortic arches. The illustrations are truly spectacular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christoffels VM, Habets PEMH, Franco D, et al. Chamber formation and morphogenesis in the developing mammalian heart. Devel Biol 2000;223:266–78. ▸ This study also combines recent observations in distribution of genes with morphological changes during development. The authors emphasise how the characteristic parts of the atriums and ventricles “balloon” from the cavities of the primary heart tube. [DOI] [PubMed] [Google Scholar]
- 13.Kim J-S, Virágh S, Moorman AFM, et al. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ Res 2001;88:395–402. ▸ An important study that re-focussed attention on the “spina vestibuli”, first described by Wilhelm His the elder in the latter part of the 19th century. The work also showed how the original musculature of the atrioventricular canal became sequestrated as the atrial vestibules subject to formation of the fibrofatty plane of insulation between the atrial and ventricular muscles masses (see also reference19). [DOI] [PubMed] [Google Scholar]
- 14.Davis CL. Development of the human heart from its first appearance to the stage found in embryos of twenty paired somites. Contrib Embryol 1927;19:245–84. ▸ It was this investigation that first demonstrated the “ballooning” of myocardium from the primary heart tube to form, in series, the apical components of the definitive left and right ventricles. [Google Scholar]
- 15.Anderson RH, Becker AE. Cardiac anatomy. An integrated text and colour atlas. London: Gower Medical Publishing, 1980:10.12–10.19. ▸ This atlas reinforced the concept of “ballooning” brought to the fore in reference12. There is a mistake in this version, however, in that the inlet of the right ventricle is shown as if developing from the inlet of the primary heart tube. In fact, as shown in reference16, the entirety of the right ventricle is derived from the outlet component of the ventricular part of the primary tube.
- 16.Lamers WH, Moorman AFM. Cardiac septation. A late contribution of the embryonic primary myocardium to heart morphogenesis. Circ Res 2002;91:93–103. ▸ An excellent review, which again puts the recent advances in molecular biology into the context of the need for a proper understanding of the morphology. [DOI] [PubMed] [Google Scholar]
- 17.Lamers WH, Wessels A, Verbeek FJ, et al. New findings concerning ventricular septation in the human heart. Implications for maldevelopment. Circulation 1992;86:1194–205. ▸ A landmark study, which used the serendipitous observation that the GlN epitope from the chick labelled the developing ventricular conduction tissues to show that the entirety of the right ventricle was derived from the outlet part of the ventricular component of the primary heart tube. [DOI] [PubMed] [Google Scholar]
- 18.van den Hoff MJB, Moorman AFC, Ruijter JM, et al. Myocardialization of the cardiac outflow tract. Devel Biol 1999;212:477–90. ▸ This work showed how pre-existing myocytes moved into the proximal parts of the fused cushions dividing the outflow tract to form the embryonic muscular outlet septum. The authors did not at this stage, however, appreciate that the embryonic outlet septum then became converted, in its greater part, to the free-standing sleeve of muscular subpulmonary infundibulum. [DOI] [PubMed] [Google Scholar]
- 19.Wessels A, Markman MWM, Vermeulen JLM, et al. The development of the atrioventricular junction in the human heart. Circ Res 1996;78:110–17. [DOI] [PubMed] [Google Scholar]