Abstract
Objective: To identify perioperative clinical predictors of permanent pacemaker implantation following aortic valve replacement.
Design and patients: Prospective cohort study on 276 patients submitted for aortic valve replacement: 267 patients (mean (SD) age, 57.5 (14) years) with no conduction disturbances, and nine patients (67.7 (5) years) with severe conduction disturbances requiring permanent pacing; 65 perioperative variables (38 preoperative, eight intraoperative, and 19 postoperative) were considered.
Results: Nine patients (3.2%) had irreversible second or third degree atrioventricular (AV) block requiring permanent pacing. Risk factors for permanent pacing identified by univariate analysis were: preoperative: additional valvar disease, aortic regurgitation, myocardial infarction, pulmonary hypertension, anaemia, use of digitalis; intraoperative: cardiac arrest; postoperative: cardiac arrest, conduction disturbances, electrolytic imbalance, angiotensin converting enzyme inhibitor use. Multivariate logistic regression analysis identified preoperative aortic regurgitation (p < 0.005; odds ratio (OR) 6.6, 95% confidence interval (CI) 1.6 to 12.2), myocardial infarction (p < 0.0005; OR 15.2, 95% CI 6.3 to 19.9), pulmonary hypertension (p < 0.005; OR 12.5, 95% CI 3.2 to 18.3), and postoperative electrolyte imbalance (p < 0.01; OR 4.5, 95% CI 1.3 to 6.4).
Conclusions: Irreversible AV block requiring permanent pacemaker implantation is an uncommon condition following aortic valve replacement. Previous aortic regurgitation, myocardial infarction, pulmonary hypertension, and postoperative electrolyte imbalance should be considered in order to identify patients at increased risk for advanced AV block.
Keywords: permanent pacemaker, aortic valve replacement, risk factors, postoperative arrhythmias
Transient conduction disorders are often encountered after heart surgery.1–11 However, the need for a permanent pacemaker is uncommon, though it significantly increases the length of hospital stay and the overall costs.4,9,12–15
The requirement for permanent pacemaker implantation (PPI) is more frequent after valve surgery (ranging from 3–6%) than after isolated coronary artery bypass grafting (0.8%).13–18 The incidence of conduction disorders requiring permanent pacing in patients operated on for aortic valve replacement has been reported to be 5.7%.16 However, perioperative risk predictors for PPI after aortic valve replacement are not well characterised.
Our aim in this study was to identify clinical predictors of postoperative PPI in a cohort of patients submitted to aortic valve replacement.
METHODS
Three hundred and one patients were operated on for aortic valve replacement at our institution (Department of Cardiothoracic Sciences, Second University of Naples) between January 2001 and January 2002. Exclusion criteria were the presence of preoperative permanent atrial fibrillation, a permanent pacemaker, and intraoperative or postoperative death during the hospital stay. According to these criteria, 276 patients entered the study. The underlying aortic diseases were: isolated aortic stenosis (n 108; 63% degenerative calcification, 31% calcification of the bicuspid valve, 6% other); isolated aortic regurgitation (n 80; 40% aortic root dilatation, 26% rheumatic disease, 15% bicuspid valve, 11% endocarditis, 8% other conditions); and combined aortic stenosis and regurgitation (n 88). A calcified aortic annulus was found in 130 patients (101 with isolated aortic stenosis, 21 with isolated aortic regurgitation, and eight with combined aortic regurgitation and stenosis).
Patients who underwent associated interventions such as other valve replacement, coronary artery bypass grafting, or left ventricular aneurysmectomy were deliberately included in the study.
For each patient, a complete preoperative echocardiographic evaluation was undertaken using an Acuson XP0 ultrasound system (Acuson Inc, Mountain View, California, USA).
Data collection was completed at the time of hospital discharge and consisted of filling out a questionnaire containing the 38 preoperative, eight intraoperative, and 19 postoperative variables (tables 1, 2, and 3). All the variables were considered to test their potential impact on the development of postoperative irreversible block requiring PPI. They included clinical characteristics of the patients (age, sex, body surface area, type of valvopathy, associated disease, and so on), drug treatment (any drugs directly affecting the conduction system, such as digitalis, or which change the pressure–volume left ventricular load or coronary blood flow, such as nitrates), surgical techniques and perioperative protocols, and postoperative complications.
Table 1.
Preoperative variables
| No PPI | PPI | p Value | |
| Age (years) | 57.5 (14) | 65.7 (5.0) | 0.08 |
| Age >70 | 25% | 22% | 0.85 |
| Male sex (%) | 61% | 67% | 0.98 |
| Height (cm) | 172.3 (4.0) | 172.0 (2.0) | 0.96 |
| Body mass index (kg/m2) | 26.2 (4.0) | 25.1 (2.0) | 0.82 |
| Body surface area (m2) | 1.73 (0.20) | 1.66 (0.10) | 0.09 |
| Previous MI (%) | 3% | 22% | 0.05 |
| Anterior location of MI (%) | 54% | 56% | 0.82 |
| Previous PTCA (%) | 0.4% | 0% | 0.79 |
| Previous CABG (%) | 0.4% | 0% | 0.79 |
| COPD (%) | 11% | 0% | 0.6 |
| Hypertension (%) | 28% | 22% | 0.98 |
| Diabetes (%) | 15% | 22% | 0.91 |
| CHD (%) | 6% | 22% | 0.22 |
| Aortic stenosis (%) | 40% | 22% | 0.45 |
| Aortic regurgitation (%) | 28% | 67% | 0.03 |
| Aortic stenosis + regurgitation (%) | 32% | 11% | 0.33 |
| Associated valve disease (%) | 25% | 78% | 0.002 |
| Calcified aortic annulus | 45% | 46% | 0.86 |
| Other cardiac disease (%) | 16% | 33% | 0.37 |
| LVEF (%) | 50.4 (13.0) | 45.6 (12.3) | 0.27 |
| NYHA class | 2.44 (0.60) | 2.61 (0.50) | 0.45 |
| Bioprosthesis (%) | 2.7% | 0% | 0.57 |
| Left atrial enlargement (%) | 61% | 75% | 0.61 |
| Pulmonary hypertension (%) | 7% | 37.5% | 0.01 |
| Preoperative anaemia (%) | 2% | 25% | 0.02 |
| Preoperative electrolytic disorders (%) | 2% | 12,5% | 0.40 |
| Syncope (%) | 5% | 12% | 0.87 |
| Premature ventricular beats (%) | 7% | 11% | 0.92 |
| Preoperative paroxysmal AF (%) | 4% | 0% | 0.79 |
| Atrioventricular conduction disorders (%) | 3% | 12% | 0.59 |
| Intraventricular conduction disorders (%) | 21% | 0% | 0.26 |
| Preoperative calcium antagonist (%) | 7% | 0% | 0.88 |
| Preoperative β blockers (%) | 4% | 0% | 0.79 |
| Preoperative digitalis (%) | 42% | 88% | 0.03 |
| Preoperative amiodarone (%) | 7% | 0% | 0.88 |
| Preoperative ACE inhibitor (%) | 41% | 75% | 0.09 |
| Preoperative nitrates (%) | 21% | 12% | 0.81 |
Values are mean (SD) or %.
ACE, angiotensin converting enzyme; AF, atrial fibrillation; CABG, coronary artery bypass graft; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PPI, permanent pacemaker implantation; PTCA, percutaneous transluminal coronary angioplasty.
Table 2.
Intraoperative variables
| No PPI | PPI | p Value | |
| Bypass time (min) | 91 (35) | 112 (59) | 0.09 |
| Ischaemia time (min) | 61 (22) | 72 (38) | 0.14 |
| IABP (%) | 0.4% | 0% | 0.79 |
| Hypothermia (%) | 83% | 100% | 0.37 |
| Warm blood cardioplegia (%) | 17% | 0% | 0.38 |
| Hyperkalaemic cold cardioplegia (%) | 83% | 100% | 0.38 |
| Intraoperative acidosis (%) | 10% | 12% | 0.71 |
| Intraoperative transfusion (%) | 8% | 11% | 0.79 |
Values are mean (SD) or %.
IABP, intra-aortic balloon pump; PPI, permanent pacemaker implantation.
Table 3.
Postoperative variables
| No PPI | PPI | p Value | |
| Inotropic agents (%) | 45 | 55 | 0.80 |
| Ventilation >24 h (%) | 4 | 7 | 0.68 |
| Postoperative acidosis (%) | 25 | 33 | 0.87 |
| Postoperative electrolytic disorders (%) | 25 | 67 | 0.009 |
| Total CK (mg/dl) (mean (SD)) | 785.4 (35.)6 | 805.4 (28.6) | 0.97 |
| MB-CK > normal range (%) | 8 | 0 | 0.80 |
| Postoperative blood transfusion (%) | 20 | 25 | 0.95 |
| Return to operating room (%) | 0.4 | 0 | 0.79 |
| Cerebral implications (%) | 1.5 | 0 | 0.29 |
| Pulmonary complications (%) | 1.5 | 0 | 0.29 |
| Cardiac implications (%) | 3 | 0 | 0.63 |
| Renal complications (%) | 1.5 | 2 | 0.20 |
| Postoperative paroxysmal AF (%) | 23 | 25 | 0.96 |
| Postoperative chronic AF (%) | 9 | 11 | 0.70 |
| Postoperative adjunctive IV conduction disorders (%) | 7 | 11 | 0.84 |
| Postoperative calcium antagonist (%) | 5 | 0 | 0.91 |
| Postoperative β blockers (%) | 3.9 | 0 | 0.77 |
| Postoperative nitrates (%) | 12 | 25 | 0.51 |
| Postoperative ACE inhibitors (%) | 34 | 88 | 0.006 |
ACE, angiotensin converting enzyme; AF, atrial fibrillation; CK, creatine kinase; MB-CK, creatine kinase, MB isoenzyme; PPI, permanent pacemaker implantation.
Surgical technique
A team of four surgeons did all the operations. Cardiopulmonary bypass with moderate haemodilution (packed cell volume 20–28%), flow rates of 22–24 l/min/m2, and a mean pressure of 50–70 mm Hg were used. Myocardial protection was achieved by the direct infusion into the coronary ostium of warm blood cardioplegia or hyperkalaemic cold crystalloid cardioplegia (St Thomas’ 1 solution). When the latter was chosen, core cooling down to 28°C was undertaken in order to achieve hypothermia. In both cases, a volume of 10 ml/kg of the solution was always given by the antegrade route at a rate of 200–250 ml/min. A further half dose of the cardioplegic solution was given every 20 or 30 minutes, depending on the type of cardioplegia adopted (respectively warm blood or cold crystalloid). The left ventricle was always vented through the right superior pulmonary vein. The aortic valve was completely excised in all cases; annulus debridement of calcium was done in a blunt fashion with forceps.
Postoperative protocols
Patients were weaned off of the ventilator as soon as they appeared haemodynamically stable, normothermic, and conscious, and with blood losses in the normal range. Potassium and magnesium supplements were provided as necessary to maintain electrolyte balance within the normal range. Electrolyte disorders were defined as serum potassium of < 3.5 mol/l, serum magnesium of < 0.82 mmol/l, and serum calcium of < 2 mmol/l. For each patient, heart rate, ECG lead II, arterial pressure, and central venous pressure were monitored continuously during their stay in the intensive care unit. Intraoperative and postoperative acidosis was defined as an arterial pH of < 7.36. A 12 lead ECG was recorded on a daily basis until discharge.
Postoperative bradyarrhythmias
Patients with symptomatic second and third degree atrioventricular (AV) block persisting more than seven days received a permanent pacemaker and were included in the PPI group (table 4), while those with self limiting bradycardia or conduction defects were placed in the non-paced (no PPI) group.
Table 4.
Patients requiring a postoperative permanent pacemaker
| Patient | Age (years) | Sex | Aortic disease | Indication for PPI | Postoperative day of PPI | Pacing mode |
| 1 | 65 | M | Stenosis | 2nd degree AV block | 8 | Dual chamber |
| 2 | 69 | M | Regurgitation | 3rd degree AV block | 9 | Dual chamber |
| 3 | 74 | F | Regurgitation | 2nd degree AV block | 9 | VVI-R |
| 4 | 61 | M | Stenosis | 3rd degree AV block | 10 | Dual chamber |
| 5 | 61 | F | Stenosis + regurgitation | 2nd degree AV block | 8 | Dual chamber |
| 6 | 63 | M | Regurgitation | 3rd degree AV block | 9 | Dual chamber |
| 7 | 60 | M | Regurgitation | 3rd degree AV block | 10 | VVI-R |
| 8 | 66 | F | Regurgitation | 2nd degree AV block | 10 | Dual chamber |
| 9 | 73 | M | Regurgitation | 3rd degree AV block | 9 | VVI-R |
Statistical analysis
Statistical analysis was undertaken using the SPSS statistical package for windows, release 8.0 (Chicago, Illinois, USA). Univariate relations of all perioperative factors with the requirement for PPI following aortic valve replacement were analysed using Student’s t test and the χ2 test: Student’s t test was used to identify significant differences in numerical variables, while categorical variables were analysed using the χ2 test. Differences were considered significant at a probability value of p < 0.05. Stepwise forwards multiple logistic analyses were done to examine the independent effects of potential predictors on the dependent variable. Univariate factors showing a value of p < 0.05 were entered into multivariate logistic regression analysis.
RESULTS
PPI was required in nine (3.2%) of the 276 patients with aortic valve replacement (table 4). Postoperative rhythm disorders requiring PPI included irreversible second or third degree AV block. In each case of pacemaker implantation, the need for single or double chamber stimulation was an important consideration. Double chamber stimulation was considered as the first option for most of the patients requiring a permanent pacemaker (patients 1, 2, 4, 5, 6, 8). However, for those with previous episodes of paroxysmal atrial fibrillation (patient 7) or with severe left or right atrial enlargement (patients 3 and 9), single chamber stimulation (VVI-R) was preferred.
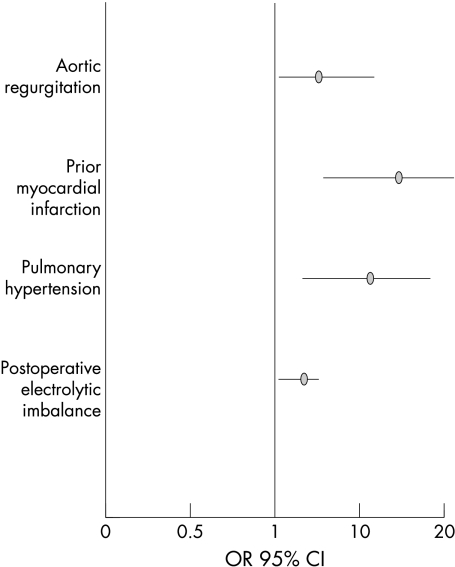
Tables 1, 2, and 3 show the differences on univariate analysis between patients with postoperative PPI and those without PPI. A multivariate logistic regression model that included all the remaining significant variables after univariate analysis was then created. Preoperative aortic regurgitation (odds ratio (OR) 6.6, 95% confidence interval (CI) 1.6 to 12.2); p < 0.005), pulmonary hypertension (OR 12.5, 95% CI 3.2 to 18.3; p < 0.005), previous myocardial infarction (OR 15.2, 95% CI 6.3 to 19.9; p < 0.0005), and postoperative electrolytic disorders (OR 4.5, 95% CI 1.3 to 6.4; p < 0.01) were all independently related to the postoperative need for PPI (fig 1).
Figure 1.
Multivariate predictors of permanent pacing requirement following aortic valve replacement in a multivariate logistic model which included all the perioperative variables. CI, confidence interval; OR, odds ratio.
DISCUSSION
Aortic valve replacement is recommended as standard management for patients with symptomatic aortic valve disease, independent of age.19–22 Following surgery, patients usually have a dramatic improvement in their cardiovascular symptoms and have improved survival rates at 5, 10, and 15 years.23,24 Persistent AV block requiring permanent pacing is an uncommon but serious postoperative complication which increases mechanical ventilation times, intensive care unit stay, and overall hospital day.12–15
Previous studies have already evaluated perioperative risk predictors of PPI after cardiac surgery in large cohorts of patients with different diseases.12–18 However, there are only scanty data on the development of irreversible AV block requiring PPI after aortic valve replacement, especially in the presence of isolated aortic regurgitation or in the case of combined aortic stenosis and regurgitation.
In our series of 276 patients (39% with isolated aortic stenosis, 29% with isolated aortic regurgitation, and 32% with combined aortic stenosis and regurgitation), we found severe heart block requiring PPI only in 3.2%. Using logistic regression analysis in a large number of patients, we identified several important clinical predictors of the need for a permanent pacemaker following aortic valve surgery (fig 1).
Aortic regurgitation was the clinical factor that was most predictive of the need for a permanent pacemaker. Aortic regurgitation may be accompanied by severe annulus ectasia which imposes chronic and progressive mechanical stretch on the nearby AV node and His bundle. In this condition, the presence of pulmonary hypertension acting on the right ventricular dimensions, shape, and interventricular septal thickness could affect the conduction system by altering the electrophysiological properties of its fibres. In addition, a previous myocardial infarction may contribute to further injury of the conduction system. On the background of structural heart disease, external triggering factors such as electrolytic disorders could play a part in the development of irreversible conduction disorders.
In the original series by Boughaleb and colleagues,16 dealing exclusively with isolated aortic stenosis, a pre-existing conduction defect, a decreased ejection fraction, and aortic annulus calcification were found to be independent predictors of PPI following aortic valve replacement. Surgical manipulation of anatomical structures near the AV node in patients with aortic stenosis and calcified aortic annulus may be a source of mechanical trauma to the conduction system, precipitating pre-existing conduction defects or generating new ones. In contrast, in our series of patients including both aortic stenosis and regurgitation, only 130 (47%) had a calcified aortic annulus. In these patients, careful debridement of annular tissue at the atrioventricular sinus area (the non-coronary cusp of the aortic valve, the posterior commissure of the anterior mitral leaflet, and the septal leaflet of the tricuspid valve annulus) was attempted in order to avoid injury to the conduction system.
Advanced irreversible AV block requiring PPI is an uncommon condition following aortic valve replacement. The development of irreversible AV block and the need for a permanent pacemaker can be identified from several clinical risk factors. These include previous aortic regurgitation, myocardial infarction, pulmonary hypertension, and postoperative electrolyte imbalance. These factor should be considered in order to identify patients at increased risk of advanced AV block.
Study limitations
Some variables (for example, age, total creatine kinase, and bypass time) with strong trends toward differences between groups may not have reached significance owing to the small sample size of the study groups.
A team of four different surgeons performed the operations. However, all the patients were treated at a single centre, and the operative methods remained substantially unchanged during the study.
REFERENCES
- 1.Chung MK. Cardiac surgery: postoperative arrhythmias. Crit Care Med 2000;28(suppl):N136–44. [DOI] [PubMed] [Google Scholar]
- 2.Sloan SB, Weitz HH. Postoperative arrhythmias and conduction disorders. Med Clin North Am 2001;85:1171–89. [DOI] [PubMed] [Google Scholar]
- 3.Baterman TM, Weiss MH, Czer LSC, et al. Fascicular conduction disturbances and ischemic heart disease: adverse prognosis despite coronary revascularization. J Am Coll Cardiol 1985;5:623–9. [DOI] [PubMed] [Google Scholar]
- 4.Emlein G, Huang SKS, Pires LA, et al. Prolonged bradyarrhythmias after isolated coronary by-pass graft surgery. Am Heart J 1993;126:1084–90. [DOI] [PubMed] [Google Scholar]
- 5.Blanche C, Czer LSC, Trento A, et al. Bradyarrhythmias requiring pacemaker implantation after orthotopic heart transplantation: association with rejection. J Heart Lung Transplant 1992;11:446–52. [PubMed] [Google Scholar]
- 6.Fournial JF, Brodaty D, Chomette G, et al. Conduction disorders after aortic valve replacement. Apropos of 200 cases. Arch Mal Coeur Vaiss 1979;72:4–11. [PubMed] [Google Scholar]
- 7.Fazzini PF, Marchi F, Salvatore L. Atrioventricular block following aortic valve replacement. Boll Ital Cardiol 1972;17:1154–6. [PubMed] [Google Scholar]
- 8.Poveda J, Vasquez J, Larman M, et al. Conduction defects in aortic valve diseases after isolated aortic valve replacement [abstract]. PACE 1983;6:A-15. [Google Scholar]
- 9.Thomas JL, Dickstein RA, Parker FB, et al. Prognostic significance of the development of left bundle conduction defects following aortic valve replacement. J Thorac Cardiovasc Surg 1982;84:382–6. [PubMed] [Google Scholar]
- 10.Keefe DL, Griffin JC, Harrison DC, et al. Atrioventricular conduction abnormalities in patients undergoing isolated aortic or mitral valve replacement. PACE Pacing Clin Electrophysiol 1985;8:393–8. [DOI] [PubMed] [Google Scholar]
- 11.Habicht JM, Scherr P, Zerkowski HR, et al. Late conduction defects following aortic valve replacement. J Heart Valve Dis 2000;9:629–32. [PubMed] [Google Scholar]
- 12.Lewis JW, Webb CR, Pickard SD, et al. The increased need for a permanent pacemaker after reoperative cardiac surgery. J Thorac Cardiovasc Surgery 1998;116:74–81. [DOI] [PubMed] [Google Scholar]
- 13.Gordon RS, Ivanov J, Cohen G, et al. Permanent cardiac pacing after a cardiac operation: predicting the use of permanent pacemakers. Ann Thorac Surg 1998;661:1698–704. [DOI] [PubMed] [Google Scholar]
- 14.Del Rizzo DF, Nishimura S, Lau C, et al. Cardiac pacing following surgery for acquired heart disease. J Card Surg 1996;11:332–40. [DOI] [PubMed] [Google Scholar]
- 15.Lipton IH, Cameron DA, David TE, et al. Morbidity of permanent pacing following valvular surgery [abstract]. PACE 1995;18:1768. [Google Scholar]
- 16.Boughaleb D, Mansourati J, Genet L, et al. Permanent cardiac stimulation after aortic valve replacement: incidence, predictive factors and long term prognosis. Arch Mal Coeur Vaiss 1994;87:925–30. [PubMed] [Google Scholar]
- 17.Goldmann BS, Hill TJ, Weisel RD, et al. Permanent cardiac pacing after open heart surgery: acquired heart disease. PACE 1984;7:367–71. [DOI] [PubMed] [Google Scholar]
- 18.Goldmann BS, Ivanov J, Irwin M, et al. Permanent pacing after cardiac surgery [abstract]. PACE 1990;13:554. [Google Scholar]
- 19.Braunwald E. Aortic valve replacement: an update at the turn of the millennium. Eur Heart J 2000;21:1032–3. [DOI] [PubMed] [Google Scholar]
- 20.Grunkemeier GL, Li HH, Starr A. Heart valve replacement: a statistical review of 35 years’ results. J Heart Valve Dis 1999;8:466–71. [PubMed] [Google Scholar]
- 21.David TE. Aortic valve surgery: where we are and where we shall go. J Heart Valve Dis 1999;8:495–8. [PubMed] [Google Scholar]
- 22.Sprigings DC, Forfar JC. How should we manage symptomatic aortic stenosis in the patient who is 80 years or older? Br Heart J 1995;74:481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kvidal P, Bergstrom R, Malm T, et al. Long term follow-up of morbidity and mortality after aortic valve replacement with a mechanical valve prosthesis. Eur Heart J 2000;21:1099–111. [DOI] [PubMed] [Google Scholar]
- 24.Kvidal P, Bergstrom R, Horte LG, et al. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000;35:747–56. [DOI] [PubMed] [Google Scholar]