Abstract
This is the first description of a case of isolated non-compaction of the left ventricular myocardium, involving a 52 year old woman presenting with progressive heart failure, with analysis of myocardial perfusion by contrast echocardiography in comparison with magnetic resonance imaging.
Keywords: non-compaction cardiomyopathy, contrast echocardiography, perfusion
Isolated non-compaction of the ventricular myocardium is a rare disorder characterised by an excessively prominent trabecular meshwork and deep intratrabecular recesses.1–3 This idiopathic cardiomyopathy is characterised by an altered structure of the myocardial wall as a result of intrauterine arrest of compaction of the myocardial fibres in the absence of any coexisting congenital lesion. There is continuity between the left ventricular cavity and the intratrabecular recesses without evidence of communication to the epicardial coronary artery system. Non-compaction cardiomyopathy was described initially in paediatric patients but recent studies have characterised it in adults.4–6
CASE REPORT
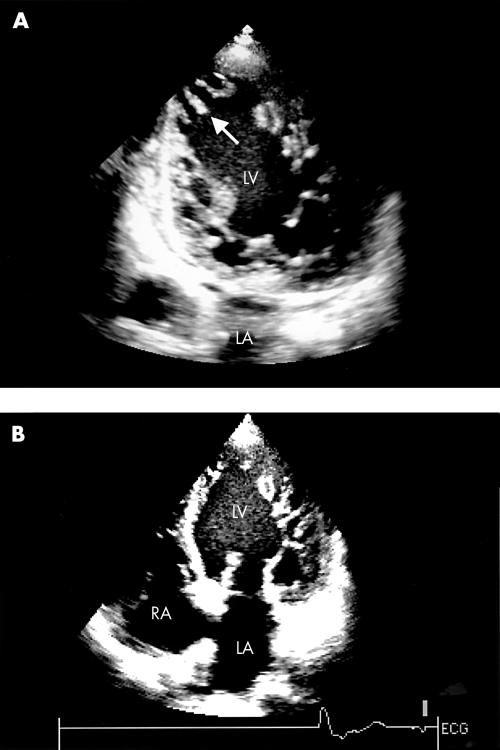
A 52 year old white woman was admitted to our institution for progressive heart failure. Transthoracic echocardiography showed increased left ventricular wall thickness and typical prominent trabeculations and intertrabecular recesses, which are pathognomonic for ventricular non-compaction (fig 1). The right ventricle was slightly enlarged, with normal trabeculations and moderately reduced systolic function. The calculated pulmonary artery systolic pressure was 50 mm Hg. Left ventricular ejection fraction was 20%. Moderate functional mitral and tricuspid regurgitation were present.
Figure 1.
Two dimensional echocardiogram: (A) apical two chamber view and (B) apical four chamber view showing numerous prominent trabeculations (arrow) and intertrabecular recesses in the apex and free wall of the left ventricle (LV). L, left atrium; RA, right atrium.
She had had no symptoms until age 48 years, when she began to have progressive left heart failure. There was no family history of cardiomyopathy or heart failure and her two daughters both had a normal echocardiogram.
The results of multiple echocardiographic examinations and left ventriculography performed two years before were interpreted as being consistent with dilated cardiomyopathy. Coronary angiography did not show any coronary anomaly or significant obstructive coronary artery disease.
Myocardial biopsy had excluded active myocarditis two years previously. Poor exercise capacity and low maximal oxygen consumption (8 ml/kg/min) were shown by treadmill exercise testing.
Holter ECG showed a non-sustained ventricular tachycardia and a permanent cardioverter-defibrillator was implanted. Because of progressive worsening of heart failure despite optimal treatment, the patient was referred for possible heart transplantation.
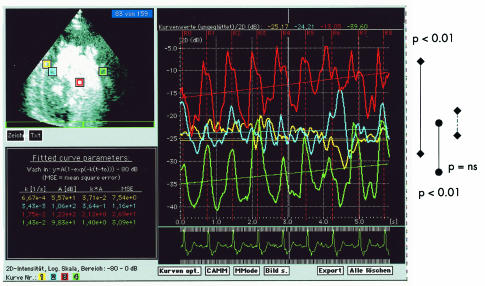
Myocardial contrast echocardiography (Vived FiVe, GE Vingmed Ultrasound, Horten, Norway; echo contrast: infusion of 3 ml Optison over four minutes; real time perfusion imaging: quantitative measurement, mechanical index 0.12) showed segmental heterogeneous segmental perfusion (higher perfusion in the middle septal segment than in the lateral wall) and transmural homogeneous perfusion (fig 2).
Figure 2.
Quantitative contrast echocardiography showing segmental heterogeneous segmental perfusion (higher perfusion in the middle septal segment shown by blue and yellow curves in comparison with the lateral wall shown by the green curve; ventricular cavum is indicated by the red curve) and transmural homogeneous perfusion (no significant differences between the septal positions: endocardial layer indicated by the blue curve and outer layer by the yellow curve).
Magnetic resonance first pass perfusion imaging was performed at 1.5 T (Magnetom Vision, Siemens, Erlangen, Germany) using a single slice T1 weighted fast gradient echo sequence in the midventricular short axis view during rapid intravenous injection of 0.05 mmol/kg gadopentetate dimeglumine (Magnevist, Schering, Berlin, Germany) at a rate of 5 ml/s. Magnetic resonance first pass perfusion imaging comprised 90 acquisitions with a temporal resolution of one image per heart beat. Semiquantitative perfusion analysis was done by computing a steepest slope parametric map using the ARGUS evaluation software (Numaris/3 WIP 2.X, Siemens Corp Research).
Magnetic resonance tomography (Magnetom Vision, Siemens, 1.5 T) confirmed the echocardiographic findings with myocardial trabeculations, showing a higher perfusion in the septum (green and yellow colour coded areas) than in the lateral wall (red) in the short axis view of the left ventricle (fig 3).
Figure 3.
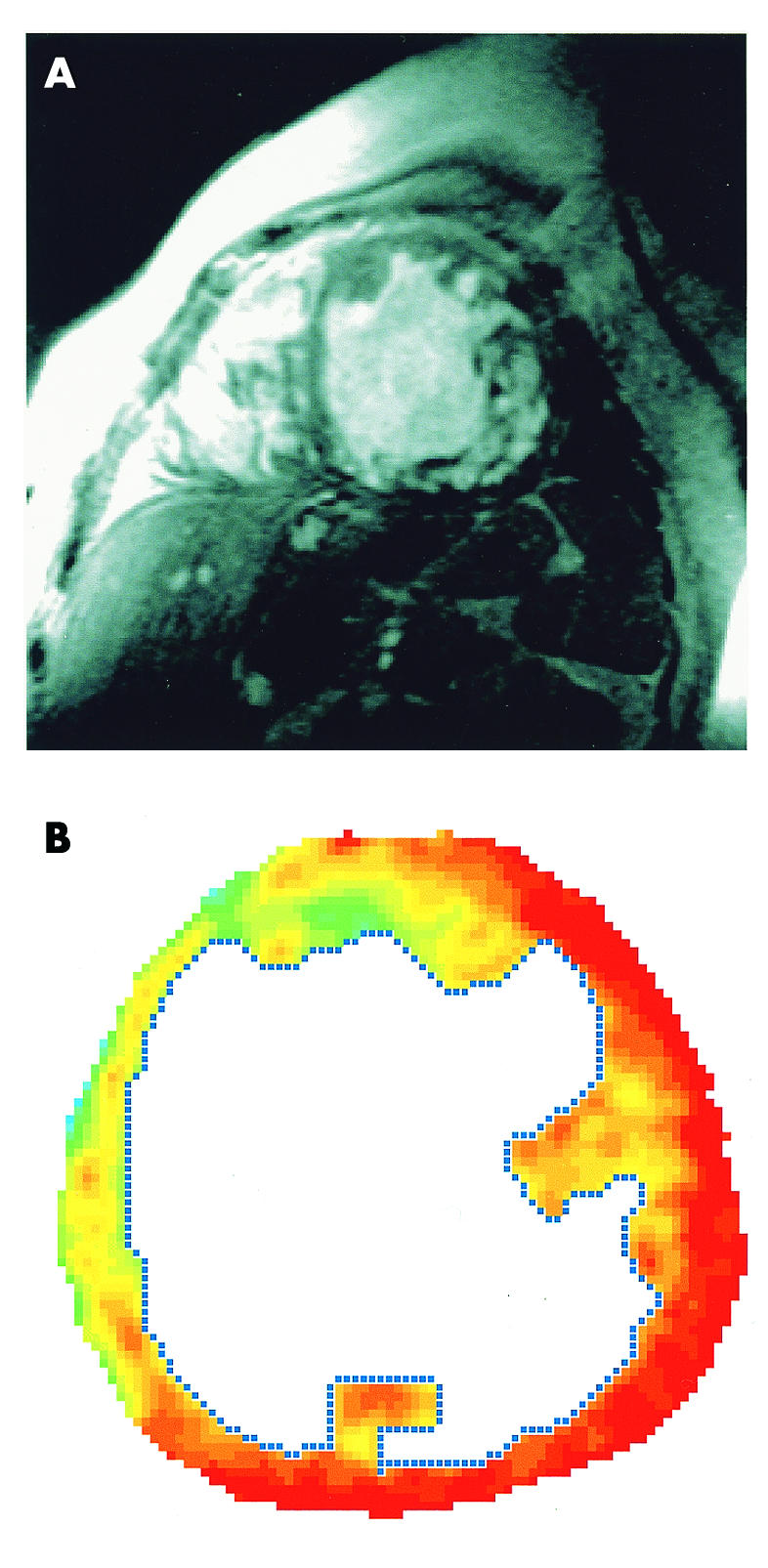
(A) Short axis view of the left ventricle showing trabeculations and intratrabecular recesses. (B) Magnetic resonance first pass perfusion imaging using a single slice T1 weighted fast gradient echo sequence in the midventricular short axis view, showing a higher perfusion rate of the septum (yellow) and a lower perfusion rate in the lateral wall (red) without a transmural gradient.
DISCUSSION
Non-compacted myocardium has been categorised as unclassified congenital cardiomyopathy and is characterised by an altered structure of the left ventricular myocardium with extremely thickened, hypokinetic segments consisting of two layers: thin, compacted myocardium on the epicardial side, and a thicker non-compacted endocardial layer. Non-compaction of the ventricular myocardium results from an arrested normal process of myocardial compaction (normally during the first month of fetal life). Right ventricular non-compaction may accompany left ventricular non-compaction in less than 50% of patients.
Mutations in the gene G4.5 on the Xq28 chromosomal region are responsible for non-compaction and result in a wide spectrum of severe infantile cardiomyopathic phenotypes, including isolated left ventricular non-compaction, as well as Barth syndrome with dilated cardiomyopathy, skeletal myopathy, neutropenia, and abnormal mitochondria. Recently, Ichida and colleagues4 described a genetic heterogeneity in non-compaction, with mutation of a novel gene, α dystrobrevin. Mutations in G4.5 result not only in Barth syndrome and left ventricular non-compaction but also in other X linked infantile cardiomyopathies, including X linked endocardial fibroelastosis.
Transthoracic echocardiography and magnetic resonance tomography are the diagnostic methods of choice for non-compaction cardiomyopathy (figs 1 and 3). Ventriculography and computed tomography may also show the typical morphological features of non-compaction: pathognomonic combination of, firstly, multiple prominent ventricular trabeculations and, secondly, multiple deep intertrabecular recesses communicating with the ventricular cavity. Most commonly, apical and midventricular segments of both the inferior and lateral wall were affected in more than 80% of patients.2 Involvement of the midventricular anterior wall and septum and the basal segments is much less frequent, and was seen also in the present case.
The morphological criteria of non-compaction are qualitative and there is subjectivity and interobserver variability in interpretation. The quantitative definition of ventricular trabeculation in non-compaction according to Chin and colleagues5 has not yet been fully accepted in clinical practice. A ratio of non-compacted to compacted myocardium of > 2 is diagnostic for non-compaction cardiomyopathy.2
Myocardial contrast echocardiography can image the microcirculation with high spatial and temporal resolution and may be useful in the diagnosis of disturbances in this rare entity, as shown is this case.
There is no specific treatment option for non-compaction cardiomyopathy and treatment includes all that is available for the treatment of heart failure. A more aggressive approach to diagnosis and treatment of ventricular arrhythmias may be justified, and prophylactic anticoagulation may be warranted because of the higher risk of thrombus formation within the intratrabecular recesses.
REFERENCES
- 1.Agmon Y, Connolly HM, Olson LJ, et al. Noncompaction of the ventricular myocardium. J Am Soc Echocardiogr 1999;12:859–63. [DOI] [PubMed] [Google Scholar]
- 2.Oechslin EN, Attenhofer Jost CH, Rojas JR, et al. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 2000;36:493–500. [DOI] [PubMed] [Google Scholar]
- 3.Hamamichi Y, Ichida F, Hashimoto I, et al. Isolated noncompaction of the ventricular myocardium: ultrafast computed tomography and magnetic resonance imaging. Int J Cardiovasc Imaging 2001;17:305–14. [DOI] [PubMed] [Google Scholar]
- 4.Ichida F, Tsubata S, Bowles K, et al. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation 2001;102:1256–64. [DOI] [PubMed] [Google Scholar]
- 5.Chin TK, Perloff JK, Williams RG, et al. Isolated noncompaction of left ventricular myocardium: a study of eight cases. Circulation 1990;82:507–13. [DOI] [PubMed] [Google Scholar]
- 6.Ritter M, Oechslin E, Sutsch G, et al. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 1997;72:26–31. [DOI] [PubMed] [Google Scholar]