Abstract
Objectives: To assess the extent of neointimal proliferation and the safety and efficacy of stent redilatation in patients with stents implanted for aortic coarctation or branch pulmonary artery stenosis.
Design: Retrospective observational study.
Setting: Tertiary referral centre.
Patients and interventions: Of 60 patients with Palmaz stents, 12 with coarctation and 12 with branch pulmonary artery stenosis (with 21 stented sites) underwent recatheterisation and stent redilatation.
Results: Neointimal thickening > 1 mm was detected in six of the 12 coarctation stents and at nine of the 21 stented sites in branch pulmonary arteries (five of which had been overdilated at implantation). Eight of the coarctation stents were electively underdilated at implantation. Coarctation stent redilatation increased median (range) lumen diameter from 9.2 (6.3–11.1) mm to 11.7 (9.8–14.1) mm (p = 0.002), decreased gradient from 10.5 (0–20) mm Hg to 0.5 (0–15) mm Hg (p = 0.008), and increased the ratio of stent diameter to descending aorta diameter from 0.66 (0.38–1.02) to 0.85 (0.52–1.2) (p = 0.008). Pulmonary artery stent redilatation increased lumen diameter from 6.9 (3.8–13.5) mm to 8.8 (4.8–14.1) mm (p < 0.001), decreased gradient from 24 (2–62) mm Hg to 12 (0–29) mm Hg (p < 0.001), and increased the ratio of stent diameter to diameter of distal pulmonary artery from 0.66 (0.44–1.5) to 0.86 (0.48–1.88) (p = 0.001). Dilatation of one peripheral pulmonary artery stent resulted in rupture of the vessel distal to the stent.
Conclusions: Neointimal proliferation is precipitated by overdilating stents at implantation. Redilatation using balloons matched to distal vessel diameter increases stent lumen size, but may not optimise vessel diameter. Redilatation is effective whether the indication for redilatation is a resistant stenosis at implantation, underexpansion at implantation, neointimal proliferation, or relative stenosis caused by growth.
Keywords: stent, aortic coarctation, pulmonary artery stenosis, redilatation
Stent implantation to treat branch pulmonary artery stenosis has rapidly gained acceptance since the procedure was first reported in 1989.1–3 Stenting for aortic coarctation is less well established, but small series have described successful dilatation of the narrow segment, reduction of gradient across the coarctation, and a low incidence of pseudoaneurysm formation.4–7 Stents placed in either of these lesions may not fully dilate the stenotic vessel at the time of implantation. This is either because the vessel wall resists further expansion8,9 or because the stent is deliberately underexpanded to reduce the risk of vessel rupture and dissection.6 Progressive neointimal proliferation may reduce the size of the stent lumen over time.2,3,10 There is also a long term concern that stent implantation may result in a fixed vessel diameter that creates an area of relative stenosis as the patient grows.11 For these reasons, it is important to establish whether stents can be redilated after they have become incorporated in the vessel wall.
The objectives of this study were: (1) to assess the extent of neointimal proliferation after stent implantation; (2) to establish whether coarctation stents and pulmonary artery stents could be redilated to optimise vessel diameter; and (3) to evaluate the safety of stent redilatation.
METHODS
Between February 1993 and March 2000, balloon expandable Palmaz stents (Cordis Europa, Roden, Netherlands) were implanted in 22 patients with aortic coarctation and 38 patients with branch pulmonary artery stenosis at a tertiary referral centre for congenital heart disease, using techniques described in previous reports.1,6,9,12,13 Coarctation stents were electively underexpanded, aiming to dilate the stent to 70–80% of the diameter of the descending aorta at the diaphragm, or to a diameter similar to that of the transverse aortic arch. Follow up catheterisation with stent redilatation was carried out in 12 of the patients with aortic coarctation and 12 of those with branch pulmonary artery stenosis. Parental or patient written informed consent was obtained in each case. All procedures were carried out under general anaesthesia
Patient population
Aortic coarctation
Demographic data on those patients who underwent stent redilatation are given in table 1. A single stent was implanted and redilated in each patient (four P308, six P4014, and two P5014 stents). The median age at stent implantation was 16.0 years (range 5.7–65.1 years) and at stent redilatation, 16.9 (7.6–66.0) years. The median time to redilatation was 1.2 (0.2–2.9) years. The median weight at stent implantation was 58.5 (17–92) kg and at stent redilatation, 62.7 (22–89.5) kg. Stents were redilated for residual hypertension in three patients, for clinical evidence of stent obstruction in one, for growth in one, and electively in seven, whose stents had been deliberately underexpanded at the time of implantation. One patient had a second redilatation, 3.5 years after the first, for continuing hypertension.
Table 1.
Details of patients undergoing coarctation stent redilation
| Patient | Age at stent | Age at first redilatation | Diagnosis | Previous surgery | Previous intervention |
| 1 | 5.7 | 7.6 | Native COA, AS | None | None |
| 2 | 9.8 | 10.7 | Native COA, supra AS | None | None |
| 3 | 45.1 | 46.8 | Native COA | None | None |
| 4 | 25.7 | 28.0 | Native COA, aneurysm AAo | None | None |
| 5 | 65.1 | 66.0 | Native COA | None | None |
| 6 | 14.7 | 14.9 | Native COA | None | None |
| 7 | 16.4 | 16.9 | Native COA, cleft AMVL | None | None |
| 8 | 14.1 | 16.9 | ReCOA | SCFlap, Dacron patch, Conduit | BDx1 |
| 9 | 34.7 | 36.8 | ReCOA | E/E | None |
| 10 | 15.5 | 16.5 | ReCOA | Dacron patch | None |
| 11 | 7.7 | 8.9 | ReCOA | SCFlap | BDx2 |
| 12 | 23.9 | 24.8 | ReCOA | Dacron patch | BDx1 |
AAo, ascending aorta; AMVL, anterior mitral valve leaflet; AS, aortic stenosis; BD, balloon dilatation; COA, coarctation of the aorta; E/E, resection and end to end anastomosis; SCFlap, subclavian flap aortoplasty; supra AS, supravalve aortic stenosis.
Pulmonary artery stenosis
Demographic data on those patients who underwent stent redilatation are given in table 2. There were 16 redilatation procedures, as three patients had a second redilatation (0.5–1.2 years after the first) and one patient had two separate redilatations (one for each branch pulmonary artery). In all, 31 stents were redilated at 21 different stenotic sites (16 P308, four P188, one P128, two P1507, three P1007, one P2006, one P1806, and three P1006 stents).
Table 2.
Details of patients undergoing pulmonary artery stent redilation
| Patient | Age at first stent | Age at first redilation | Diagnosis | Site of PA stents | Previous surgery | Previous intervention |
| 1 | 13.3 | 13.8 | ToF | R+L | RBTS, LmBTS, Cshunt | BD PAs |
| 2 | 6.5 | 8.4 | ToF | L | RmBTS, repair ToF | BD RVOT |
| 3 | 9.4 | 15.6 | ToF | R | LBTS, RmBTS, repair ToF, patch RPA+LPA | Multiple BD PAs |
| 4 | 6.3 | 7.3 | PAT+VSD+MAPCAS | L | LmBTS, unifocalisation R MAPCAS | RF perforation + balloon RVOT |
| 5 | 16.8 | 18.4 | PAT+VSD+MAPCAS | R+L | RmBTS, Brock, LmBTS, division L MAPCA | BD RVOT, BD LPA+ RPA, myectomy RVOT |
| 6 | 0.5 | 1.1 | CAT | R+L | CAT repair | None |
| 7 | 0.4 | 1.3 | CAT | R+L | CAT repair | None |
| 8 | 7.5 | 8.1 | Alagille’s syndrome | L | None | None |
| 9 | 7.6 | 9.0 | Alagille’s syndrome | R+L | None | Multiple BD PAs |
| 10 | 6.0 | 6.2 | Alagille’s syndrome | L | None | None |
| 11 | 13.1 | 15.6 | Congenital RPA stenosis, VSD, ASD | R | None | None |
| 12 | 20.6 | 22.1 | Congenital branch PA stenoses | L | None | None |
ASD, atrial septal defect; BD, balloon dilatation; Brock, Brock pulmonary valvotomy; CAT, common arterial trunk; Cshunt, central shunt; L, left; LBTS, left classical Blalock-Taussig shunt; LmBTS, left modified Blalock-Taussig shunt; LPA, left pulmonary artery; MAPCA, major aortopulmonary collateral artery; PA, pulmonary artery; PAT, pulmonary atresia; R, right; RBTS, right classical Blalock-Taussig shunt; RF, radiofrequency; RmBTS, right modified Blalock-Taussig shunt; RPA, right pulmonary artery; RVOT, right ventricular outflow tract; ToF, tetralogy of Fallot; VSD, ventricular septal defect.
The median (range) age at stent implantation was 8.3 (0.4–20.6) years and at stent redilatation, 9.7 (1.1–22.1) years. The median time to redilatation was 1.2 (0.2–6.2) years. The median weight at stent implantation was 23 (4.9–74) kg and at stent redilatation, 26.5 (7.7–73) kg. Five redilatation procedures were carried out to optimise haemodynamics before cardiac surgery, five to improve elevated right ventricular pressure, two to allow for growth, and four were elective. Two elective redilatation procedures were carried out because the patients had resistant stenoses that had caused incomplete stent expansion at implantation. Two patients were electively recatheterised to evaluate both the stents and residual pulmonary artery narrowing beyond the stented sites and were found to require redilatation.
Stent redilatation
Aortic coarctation
Access was gained through a femoral artery and a withdrawal pressure gradient was obtained across the stent. An aortogram was done in anteroposterior and lateral projections, using a 5 French marker pigtail catheter (Cook Australia, Queensland, Australia), positioning the 1 cm markers across the coarctation site to allow accurate calibration of the angiographic image. An end hole catheter was advanced across the stent and a 0.035 inch (0.9 mm) Amplatz extra stiff guidewire (William Cook Europe, Bjaeverskov, Denmark) was placed either in the ascending aorta or in a subclavian artery. A balloon catheter with a balloon length greater than or equal to the length of the expanded stent and a diameter approximating the diameter of the descending aorta at the diaphragm was advanced over the wire. High pressure balloons were used where possible (n = 6) and standard pressure balloons were used only when the desired balloon diameter exceeded the available size of high pressure balloon (n = 6). The balloon was inflated up to the manufacturer’s recommended burst pressure with a controlled pressure inflation device. Pressure was sustained for up to 30 seconds. The gradient across the stent was remeasured and the aortogram was repeated to obtain stent dimensions.
Pulmonary artery stenosis
Access was gained through a femoral vein, and right heart catheterisation was performed. Simultaneous right ventricle and systemic pressures were recorded. Redilatation was carried out with a balloon passed over an Amplatz guidewire, obtaining pressure data and acquiring angiograms before and after redilatation. The diameter of the balloon was chosen to approximate the maximum diameter of the pulmonary artery distal to the stent. High pressure (n = 16) and standard pressure (n = 5) balloons were used. If adequate stent redilatation was not achieved, a larger balloon was used. When stents had been implanted in both the right and left proximal branch pulmonary arteries or were positioned in adjacent distal branch pulmonary arteries, redilatation was carried out by simultaneous balloon inflation, to avoid balloon rupture and to prevent compression of one stent during redilatation of another. In some redilatation procedures, where there was stenosis in the branch pulmonary artery segment adjacent to the stent, further stents were implanted.
Data collection
Angiograms taken at the time of stent implantation and redilatation were analysed to determine changes in the diameter of the stent lumen. The minimum diameter of the lumen was obtained by measuring the narrowest point in the column of contrast within the stent. In the majority of coarctation cases, angiographic measurements were corrected for magnification using the calibration markers on the pigtail catheter. In pulmonary artery stenosis cases, angiographic measurements were calibrated using the known angiographic catheter diameter. In some cases measurements were made prospectively using electronic callipers. In cases where prospective data were not available, measurements were made retrospectively from a projected still frame of the angiogram, using hand held digital callipers. In coarctation cases the diameter of the descending aorta at the diaphragm was measured, to provide an index diameter for aortic size. In pulmonary artery stenosis cases the maximum diameter of the pulmonary artery distal to the stent was measured, to provide an index diameter for pulmonary artery size. The degree of lumen narrowing caused by neointimal proliferation was assessed by the separation of the column of contrast from the stent mesh. Where two planes were available, diameter measurements were made in both planes and the smallest measurement was recorded. Angiographic measurements were made at the point in the cardiac cycle where vessel diameter was maximal. Records and pressure tracings taken at the time of cardiac catheterisation were reviewed to determine pressure measurements and gradients. Patient records were reviewed to derive demographic and outcome data.
Statistical analysis
Variables are expressed as median values with the range given in parentheses (the data were non-parametric). Datasets were compared using the Wilcoxon signed rank test. A probability value of p < 0.05 was considered significant.
RESULTS
Neointimal proliferation
Aortic coarctation
A neointimal layer (> 1 mm thickness) was detectable in six of the 12 aortic stents at the time of redilatation. In two cases the neointima occurred at the site of residual waisting in the body of the stent, in two cases it formed in the body of the stent with no clear precipitating factor, and in two cases there was a neointimal ridge at the top of the stent where the transverse aortic arch turned acutely into the stented aortic isthmus, so that flow around a sharp angle became flow around a curve formed by neointima. In these cases, neointima caused a median lumen narrowing of 2.3 (1.8–3.3) mm and a median percentage reduction in stent lumen diameter of 22% (17–30%). In three cases the neointimal thickness did not change after stent redilatation. In three other cases the neointimal layer reduced in thickness by 0.2 to 1.7 mm after redilatation. There was a significant (> 10 mm Hg) increase in the gradient across the stent between implantation and recatheterisation in only one case.
Pulmonary artery stenosis
Neointimal proliferation (> 1 mm thickness) was detectable at nine of the 21 stenotic sites. In eight cases the neointimal layer was at the distal end of a stent. In five of these cases the stent had been overdilated at implantation, so that its size was greater than the size of the adjacent vessel lumen, in one case there was no clear precipitating factor, and in two cases the angiograms at implantation were not available for review. In one case neointimal proliferation occurred at the point of overlap between two stents. The stents were short and overlap was over less than 20% of their length. In the cases with neointima, median lumen narrowing was 2.6 (1.2–3.8) mm and median percentage reduction in stent lumen diameter was 31% (15–57%). After redilatation the neointimal layer reduced in thickness by a median of 1.1 (0.1–2) mm. It was possible to compare the stent gradient at implantation with the stent gradient at follow up catheterisation in seven of the nine cases. There was a significant (> 10 mm Hg) increase in gradient in four.
Stent redilatation
Aortic coarctation
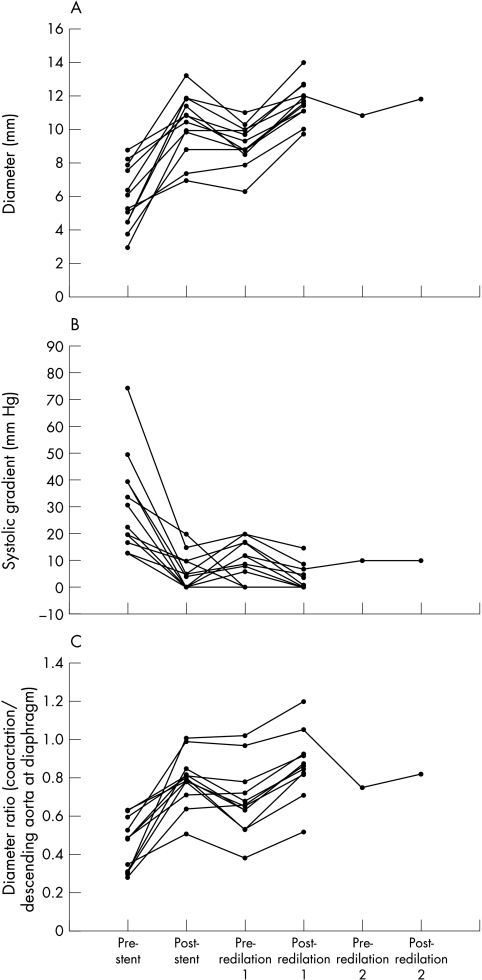
Data obtained at stent implantation and redilatation are presented in fig 1. In table 3, measurements before and after stent implantation are compared (to demonstrate the efficacy of stenting), measurements after stent implantation are compared with those before stent redilatation (to assess the degree of restenosis that developed between implantation and redilatation), and measurements before and after redilatation are compared (to assess the efficacy of redilatation). At the time of redilatation, the median increase in stent lumen diameter was 2.5 (0.1–3.8) mm and the median percentage increase in stent lumen diameter was 29% (1–56%).
Figure 1.
(A) Minimum lumen diameter at the coarctation site after stent implantation and redilatation. (B) Systolic gradient across the coarctation site after stent implantation and redilatation. (C) Ratio of minimum coarctation lumen diameter/diameter of the descending aorta at the diaphragm.
Table 3.
Coarctation: comparison of data before and after stent implantation and redilation
| Pre-stent | Post-stent | Pre-redilatation | Post-redilatation | |
| Lumen diameter (mm) | 5.7 (3.0– 8.0) | 10.7 (7.0–13.3) | 9.2 (6.3–11.1) | 11.7 (9.8–14.1) |
| n=12; p=0.002 | n=12; p=0.008 | n=12; p=0.002 | ||
| Gradient (mm Hg) | 27 (13–75) | 4.5 (0–20) | 10.5 (0–20) | 0.5 (0–15) |
| n=12; p=0.002 | n=12; p=0.15 | n=12; p=0.008 | ||
| Diameter/DAo | 0.49 (0.28–0.63) | 0.81 (0.51–1.01) | 0.66 (0.38–1.02) | 0.85 (0.52–1.20) |
| n=12; p=0.002 | n=12; p=0.01 | n=12; p=0.005 |
Median values and ranges are given for each measurement. Data are compared with those in the preceding column. The number of measurements included in the comparison and the statistical significance of changes are indicated.
Diameter/DAo, ratio of coarctation lumen diameter indexed to the diameter of the descending aorta at the diaphragm.
Measurements after stent redilatation were also compared with those after stent implantation (to assess whether there had been any real improvement on the diameter achieved at the first procedure). Successful stent expansion at the time of redilatation did not necessarily represent enlargement of the original stent lumen because in some cases redilatation only removed narrowing caused by neointimal proliferation. Median lumen diameter increased from 10.7 (7.0–13.3) mm after implantation to 11.7 (9.8–14.1) mm after redilatation (n = 12; p = 0.002). The median increase in stent lumen diameter was 1.1 (−0.5 to 2.8) mm. Changes in the systolic gradient across the stent and the ratio of coarctation lumen diameter indexed to the diameter of the descending aorta at the diaphragm (diameter/DAo) were not significant. The median ratio of final stent diameter to redilatation balloon diameter (calculated to assess whether expansion was optimal) was 0.81 (0.64–0.98).
In two patients it was not possible to expand the stent to the desired diameter at initial implantation. Redilatation achieved a 2.7 mm increase in diameter in one case and a 3 mm increase in the other (in one case the lumen size after redilatation was only slightly greater than the lumen size at implantation because of neointimal proliferation). In eight patients the aortic stent was electively underdilated at implantation. In this subgroup, redilatation increased stent diameter by a median of 1 (0.5–2.8) mm beyond the diameter achieved at implantation. In these patients, the median diameter/DAo ratio was 0.85 (0.71–0.88) after redilatation. In the six cases with > 1 mm neointimal proliferation, redilatation achieved an increase in lumen size that exceeded the amount of stenosis caused by the neointimal layer. There was significant growth between stent implantation and redilatation (defined as > 1 mm increase in the diameter of the descending aorta at the diaphragm) in only two of the 12 patients. Both patients developed mild relative stenosis caused by the stent. In one patient after a 50% increase in weight, there was a 3 mm (34%) increase in the diameter of the descending aorta at the diaphragm. The diameter/DAo ratio was 0.8 at stent implantation, 0.53 before redilatation, and 0.83 after redilatation (3.5 mm diameter increase). In the other patient, after an increase in weight of 19%, the descending aorta diameter increased by 4 mm (29%). The diameter/DAo ratio was 0.78 at stent implantation, 0.54 before redilatation, and 0.71 after redilatation (3.1 mm diameter increase).
After redilatation, median follow up time was 8.6 (0–25) months. Eleven of the 12 patients were hypertensive before stent implantation, seven remained hypertensive after stent implantation, three remained hypertensive after stent redilatation, and one patient whose hypertension had resolved after stent implantation was again found to be hypertensive 16 months after redilatation. Three of the 12 patients were treated with antihypertensive drugs before stent implantation, five required antihypertensive treatment after stenting, and five required antihypertensive treatment after stent redilatation. After stent redilatation, antihypertensive treatment was decreased in two patients and increased in one.
Pulmonary artery stenosis
Changes in vessel diameter and gradient were documented for each stenotic site, rather than for each individual stent. Where stents overlapped they were considered as one stenotic site and the minimum diameter measurement indicated the minimum diameter within the stent complex. Changes in the ratio of right ventricular systolic pressure to systemic systolic pressure (RV/Ao pressure) were calculated for eight of the 12 patients (four patients with a ventricular septal defect were excluded).
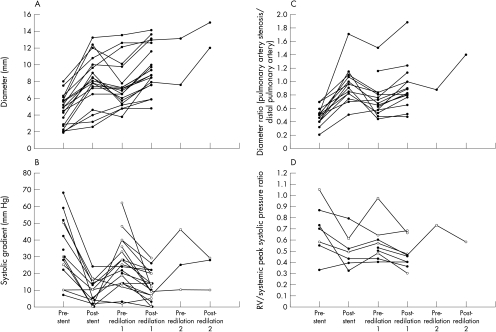
Data obtained at stent implantation and redilatation are presented in fig 2. In table 4, measurements are compared in the same way as for coarctation. At the time of redilatation, the median increase in stent lumen diameter was 1.9 (0–4.7) mm and the median percentage increase in stent lumen diameter was 28% (0–124%).
Figure 2.
(A) Minimum lumen diameter at the site of pulmonary artery stenosis after stent implantation and redilatation. (B) Systolic gradient across the site of pulmonary artery stenosis after stent implantation and redilatation. Empty circles indicate cases where haemodynamics may have been affected by: (1) further stents implanted proximal or distal to the existing stents at the time of redilatation; (2) concurrent stent implantation, stent redilatation, or balloon angioplasty at a different site in the pulmonary arteries. (C) Ratio of pulmonary artery stenosis diameter/diameter of the distal pulmonary artery. (D) Ratio of right ventricular systolic pressure/systemic systolic pressure after pulmonary artery stent implantation and redilatation. Cases with ventricular septal defect are omitted. Empty circles indicate cases where haemodynamics may have been altered by concurrent interventional procedures, as in panel B.
Table 4.
Pulmonary artery stenosis: comparison of data before and after stent implantation and redilation
| Pre-stent | Post-stent | Pre-redilatation | Post-redilatation | |
| Lumen diameter (mm) | 4.9 (1.9–8.0) | 8.1 (2.6–13.2) | 6.9 (3.8–13.5) | 8.8 (4.8–14.1) |
| n=19; p<0.001 | n=19; p=0.28 | n=19; p<0.001 | ||
| Gradient (mm Hg) | 30 (7–68) | 10 (0–24) | 24 (2–62) | 12 (0–29) |
| n=13; p=0.002 | n=13; p=0.009 | n=19; p<0.001 | ||
| Diameter/PA | 0.5 (0.21–0.7) | 0.92 (0.51–1.7) | 0.66 (0.44–1.5) | 0.86 (0.48–1.88) |
| n=15; p<0.001 | n=12; p=0.005 | n=16; p=0.001 | ||
| RV/Ao | 0.7 (0.33–1.05) | 0.49 (0.32–0.79) | 0.53 (0.4–0.97) | 0.45 (0.3–0.68) |
| n=7; p=0.28 | n=7; p=0.17 | n=9; p=0.017 |
Median values and ranges are given for each measurement. Data are compared with those in the preceding column. The number of measurements included in the comparison and the statistical significance of changes are indicated.
PA, pulmonary artery; RV/Ao, ratio of right ventricular systolic pressure to systemic systolic pressure.
Measurements after stent redilatation were again compared with those after stent implantation to evaluate whether stents had been successfully dilated beyond their implantation diameter. Median stent lumen diameter increased from 8.1 mm after implantation to 8.8 mm after redilatation (n = 19, p = 0.001). The median increase in lumen diameter was 1.7 (−1.1 to 3.9) mm. Changes in the systolic gradient across the stent, in the ratio of pulmonary artery stenosis diameter indexed to distal pulmonary artery diameter (diameter/PA), and in RV/Ao pressure were not significant. The median ratio of final stent diameter to redilatation balloon diameter was 0.79 (0.54–1.08).
Two patients had resistant stenoses that caused incomplete stent expansion at implantation. In each case there was a small increase in stent size (0.7–1.4 mm) after redilatation. In six of the cases with neointimal proliferation, redilatation achieved an increase in lumen size that exceeded the amount of stenosis caused by the neointimal layer, in two cases it did not, and in one case a complete set of measurements could not be made. Three patients who grew significantly between stent implantation and redilatation had evidence of relative stenosis at the stented site. In each of these cases the stents were successfully redilated to a diameter > 80% of the diameter of the distal pulmonary artery (2.1–3.5 mm increase in lumen size).
After redilatation, the median follow up time was 22 (5–63 months). Most patients did not have heart related symptoms before the procedure. Of four patients who had fatigue and dyspnoea on exertion, exercise tolerance improved in one, remained unchanged in two, and worsened in one on follow up after stent redilatation. Three patients had surgical correction following redilatation.
Complications
Aortic coarctation
Very few complications were encountered. One 66 year old patient had an extensive cerebral infarct during aortic stent redilatation. Further investigation revealed significant bilateral carotid artery occlusive disease. Cerebral ischaemia had occurred as a consequence of the pre-existing carotid artery stenosis and anaesthetic related hypotension documented at the start of the redilatation procedure. In another patient, a small dissection flap was noted at the top end of the stent immediately after redilatation. The flap was not detectable on computed tomography the following day. One patient also had transient bradycardia with ST segment changes during balloon inflation.
Pulmonary artery stenosis
Three patients had complications following redilatation of pulmonary artery stents.
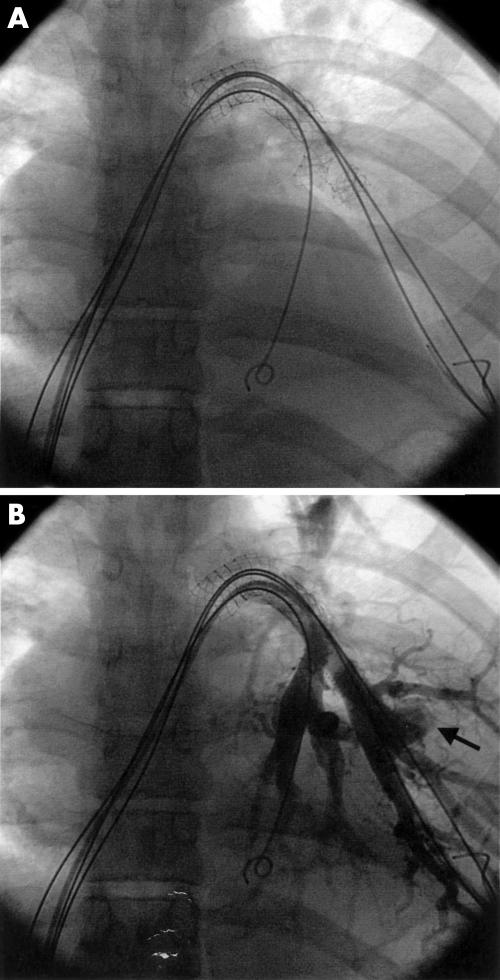
One patient with multiple congenital branch pulmonary artery stenoses of unknown origin had redilatation of three stents placed in adjacent small distal branch pulmonary arteries. Redilatation caused branch pulmonary artery disruption distal to one of the stents, with formation of a pseudoaneurysm (fig 3). Although the patient was initially stable, there was massive haemothorax with hypovolaemia 12–24 hours after the procedure, requiring emergency lobectomy. One patient developed mild pulmonary oedema because of reperfusion injury (two additional stents were implanted proximal to the redilated site). Symptoms resolved in 48 hours. In another patient a large aneurysm was discovered in the distal left pulmonary artery two years after redilatation of a stent in the proximal left pulmonary artery. The patient had Q fever endocarditis and there was no evidence that the aneurysm was related to the redilatation.
Figure 3.
Multiple stents in congenital branch pulmonary artery stenosis (A) before redilatation, and (B) after redilatation, demonstrating pseudoaneurysm formation (arrow) secondary to distal vessel damage.
DISCUSSION
Neointimal proliferation
Stents in the cardiovascular system are normally covered by a thin layer of neointima within six months of implantation.3,10,14–18 A 1–2 mm reduction in lumen diameter caused by neointima has been regarded as a normal finding.3,19 A greater degree of neointimal proliferation may cause restenosis. There was a high rate of restenosis in the pulmonary artery stents that were redilated, compared with other series which have reported significant lumen narrowing in only 1.5–3% of patients.3,10,19,20 The incidence of restenosis in those of the initial 38 patients with pulmonary artery stents who did not undergo redilatation was not evaluated. It is not therefore possible to determine the exact percentage of patients with pulmonary artery stents affected by restenosis using our data. It has been suggested that overdilating stents, minimal stent overlap, and sharp angulation of the stent to the vessel wall are risk factors for neointimal hyperplasia.2,19 Our results support the proposition that overdilatation is a risk factor, as the majority of stents that developed neointimal hyperplasia in the pulmonary artery stenosis group had been dilated beyond the diameter of the adjacent vessel at implantation. Overdilatation is the most likely explanation for the high restenosis rate. Although the incidence of restenosis in the coarctation group was comparable to that in the pulmonary artery stenosis group, the reason for restenosis was different. In pulmonary artery stenting, neointima may remodel the stent lumen to achieve a uniform vessel diameter and a smooth wall contour.3,10 When restenosis was observed in coarctation stents, it appeared to have been caused by such remodelling in most cases, as neointima formed around the site of residual waisting and at points of sudden change in flow direction.
Stent redilatation
The indications for redilating previously implanted stents include the following:
a stenosis which initially prevents optimal stent expansion
elective underexpansion at the time of implantation
neointimal proliferation within the stent that significantly reduces lumen size
growth that results in relative stenosis.
The safety and efficacy of stent redilatation for each of these indications requires assessment.
Previous studies have shown that pulmonary artery stents can be successfully redilated.2,3,10,19 Our results are not as encouraging as those from other series, which describe an increase in mean stent lumen diameter of 3–4 mm following pulmonary artery stent redilatation.2,19 Although these series showed an increase in stent size and a decrease in stent gradient following redilatation, it is not clear whether stent lumen was optimised. There are very little published data on redilatation of coarctation stents (five cases previously reported by Magee and colleagues are included in our patient group).5,6,8,19 In our study, redilatation achieved an increase in the stent diameter and a decrease in stent gradient in almost all cases. However, in many cases the stent lumen had reduced between the time of implantation and redilatation (figs 1A and 2A), so that much of the redilatation was necessary simply to restore the original lumen size.
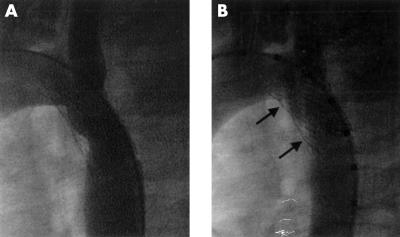
There may be a process of vessel wall remodelling after the original stent implantation, making a subsequent increase in stent diameter possible, as none of the cases required a higher pressure balloon to achieve the extra dilatation. This observation is supported by another study, which noted that further increases in stent diameter could be obtained at redilatation, although the balloon size and pressure used for redilatation was equal to that used at implantation.10 Where coarctation stents were electively underdilated at implantation, redilatation achieved a lumen size larger than that at implantation in all cases (indication (b) above), but none of the stents were expanded to the size of the descending aorta and the median increase in diameter was only 1 mm. In the majority of cases, redilatation was effective to remove any lumen narrowing caused by neointimal proliferation (indication (c) above). This confirms the findings of previous studies.2,10,21 Most of the increase in stent lumen was achieved by increasing the diameter of the stent itself, so that the stent sometimes bulged at the site of the neointimal ridge (fig 4), but part of the increase was also achieved by squeezing or flattening the neointima in some patients.
Figure 4.
Neointimal narrowing in a coarctation stent (A) before redilatation, and (B) after redilatation. As the vessel lumen has enlarged, the stent has expanded so that it bulges at the site of the neointimal ridges (arrows).
The critical question is whether a stent implanted in a young child can be redilated many years later to allow for growth (indication (d) above). As there were only a few patients with relative stenosis caused by growth, it is impossible to draw any conclusions from our results about the predictability of redilatation in these circumstances. However, our results show that such redilatation is possible. Animal studies show that stents can be successfully redilated to treat relative stenosis caused by growth, both in the aorta and in the pulmonary artery.14–18 A recent clinical study involving a large cohort of patients showed effective redilatation of stents in the pulmonary artery, where the most common indication for redilatation was growth.19 All of this evidence suggests that successful redilatation can be achieved several years after implantation, to allow for increases in body size. However, it is still uncertain whether redilatation can succeed many years after implantation, whether multiple sequential redilatations will be effective following stent implantation at a young age, and whether such redilatations can ultimately achieve a vessel lumen of adult size.
Limitations to redilatation
Redilatation may be limited either by the mechanical properties of the stent or by the capacity of the stented vessel to expand. P308 stents implanted into branch pulmonary arteries can be expanded to 18 mm.2,9 The mechanical properties of the P308 stent should therefore allow its redilatation to the size of the adult pulmonary artery, which has a diameter of approximately 18–22 mm.22 P4014 and P5014 stents can be dilated to a diameter of 25 mm. This should allow redilatation to the size of an adult aorta, which has a mean diameter of 21 (±5.5) mm at 21–40 years of age.23 The design of the stent is not therefore the limiting factor in redilatation.
Stents in our series were on average redilated to only 85–86% of the size of the distal vessel and 79–81% of the size of the balloon used for redilatation. This failure to optimise stent diameter may indicate that no further expansion was possible. Further dilatation may have been prevented by fibrosis, limited distensibility or limited tissue at the stenotic site. However, one recent series showed that coarctation stents could be expanded to the diameter of the descending aorta at implantation, but this required balloons larger than the intended stent diameter in many cases.8 It is therefore possible that further redilatation could have been obtained by using larger balloons. Two high pressure balloons side by side may also be effective. These strategies may involve an increased risk of vessel rupture, so covered stents should be available if such aggressive dilatation is undertaken. Our results show that the gradient across many of the coarctation stents was very low after redilatation, despite the fact that most stents were not expanded to the full diameter of the descending aorta. Although this is encouraging, a residual gradient may be unmasked by administering isoprenaline (isoproterenol), suggesting that failure to match the diameter of the stent to the diameter of the distal vessel may cause exercise related gradients, which could result in exercise induced hypertension. In the absence of further data, we suggest that the safest approach remains to attempt to match stent lumen to distal vessel size.
Complications
One of the most serious potential complications of stent redilatation is vessel disruption. Initial work in animal models suggested that this might be a significant risk, as one study on stents in the pulmonary artery demonstrated adventitial or extravascular stent location following redilatation and another study on experimental coarctation described immediate and fatal aortic rupture during repeat stent expansion.17,18 Despite these concerns, vessel disruption has not occurred in clinical studies that have evaluated redilatation of stents implanted for coarctation or pulmonary artery stenosis.2,3,5,6,8,10,19 However, in our study, pulmonary artery rupture occurred beyond the stent in one patient, causing massive haemorrhage. It is likely that this occurred because a long balloon was placed across a short distal stent and the balloon projected into and overdilated a small distal pulmonary artery. This complication has been described in relation to the balloon milking distally during stent dilatation.3 There is still no case of stent expansion itself causing either acute rupture or pseudoaneurysm formation after redilatation.
Recommendations
While concerns over limitations to redilatation remain, caution should be exercised when selecting young children for stent implantation, as they will inevitably require multiple redilatations to prevent relative stenosis caused by growth. Shorter balloons and great care should be used in redilating stents in small distal branch pulmonary arteries. If there is a rupture it may be preferable to insert a covered stent at the time of the procedure. We also now recommend carotid Doppler studies in all patients over the age of 50 before coarctation stenting or redilatation is carried out. As redilatation was found to be effective in expanding electively underdilated stents in the aorta, we suggest staged dilatation of coarctation stents to reduce the risk of rupture at implantation. When stents have been optimally dilated at implantation, patients can be followed up clinically and with echocardiography. If there is clinical evidence of restenosis (such as systemic hypertension or increasing right ventricular pressure) and stent gradients cannot be determined by echocardiography, lung perfusion scanning and magnetic resonance imaging can be used to identify whether restenosis has occurred, to assist in selecting patients for recatheterisation and possible redilatation.
Study limitations
A limitation of the study was the small numbers of children involved and the short time period between stent implantation and redilatation, making it difficult to assess whether redilatation could successfully treat relative stenosis caused by growth. The dataset was not complete in all patients as the study was retrospective and full information on procedures many years earlier was not always available. In the pulmonary artery stenosis group, changes in pressure gradients should be interpreted with caution as multiple stenotic sites were sometimes redilated in the same procedure and additional stents were often implanted at the time of the redilatation, changing pulmonary artery flow dynamics and right ventricular afterload. The accuracy of measurements derived from angiography was limited by difficulties in defining the edge of the stent or contrast column and the calibration mechanism used to correct for magnification. These difficulties were particularly evident when carrying out small measurements such as neointimal thickness. The anatomy of the distal pulmonary artery was variable, making it difficult to define a standard site for measurement. This made measurements of the ratio between pulmonary artery stenosis and distal pulmonary artery diameter less reproducible. Finally, the retrospective nature of the study potentially introduced bias into the measurements.
Conclusions
Stent redilatation is effective in increasing stent lumen size and decreasing gradient in cases of aortic coarctation and pulmonary artery stenosis. Redilatation is successful in treating restenosis cased by neointimal proliferation and can increase the size of a stent that is electively underexpanded at the time of implantation. Isolated cases suggest that redilatation can treat relative stenosis at the stent site caused by growth. Stent redilatation is safe in the majority of cases, although there remains a small possibility of vessel rupture.
REFERENCES
- 1.O’Laughlin MP, Perry SB, Lock JE, et al. Use of endovascular stents in congenital heart disease. Circulation 1991;83:1923–39. [DOI] [PubMed] [Google Scholar]
- 2.Fogelman R, Nykanen D, Smallhorn JF, et al. Endovascular stents in the pulmonary circulation. Clinical impact on management and medium-term follow-up. Circulation 1995;92:881–5. [DOI] [PubMed] [Google Scholar]
- 3.Shaffer KM, Mullins CE, Grifka RG, et al. Intravascular stents in congenital heart disease: short- and long-term results from a large single-center experience. J Am Coll Cardiol 1998;31:661–7. [DOI] [PubMed] [Google Scholar]
- 4.Thanopoulos BD, Hadjinikolaou L, Konstadopoulou GN, et al. Stent treatment for coarctation of the aorta: intermediate term follow up and technical considerations. Heart 2000;84:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall AC, Perry SB, Keane JF, et al. Early results and medium-term follow-up of stent implantation for mild residual or recurrent aortic coarctation. Am Heart J 2000;139:1054–60. [DOI] [PubMed] [Google Scholar]
- 6.Magee AG, Brzezinska-Rajszys G, Qureshi SA, et al. Stent implantation for aortic coarctation and recoarctation. Heart 1999;82:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez de Lezo J, Pan M, Romero M, et al. Immediate and follow-up findings after stent treatment for severe coarctation of aorta. Am J Cardiol 1999;83:400–6. [DOI] [PubMed] [Google Scholar]
- 8.Hamdan MA, Maheshwari S, Fahey JT, et al. Endovascular stents for coarctation of the aorta: initial results and intermediate-term follow-up. J Am Coll Cardiol 2001;38:1518–23. [DOI] [PubMed] [Google Scholar]
- 9.O’Laughlin MP, Slack MC, Grifka RG, et al. Implantation and intermediate-term follow-up of stents in congenital heart disease. Circulation 1993;88:605–14. [DOI] [PubMed] [Google Scholar]
- 10.Ing FF, Grifka RG, Nihill MR, et al. Repeat dilation of intravascular stents in congenital heart defects. Circulation 1995;92:893–7. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal E, Qureshi SA. Stent implantation in congenital heart disease. Br Heart J 1992;67:211–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebeid MR, Prieto LR, Latson LA. Use of balloon-expandable stents for coarctation of the aorta: initial results and intermediate-term follow-up. J Am Coll Cardiol 1997;30:1847–52. [DOI] [PubMed] [Google Scholar]
- 13.Bulbul ZR, Bruckheimer E, Love JC, et al. Implantation of balloon-expandable stents for coarctation of the aorta: implantation data and short-term results. Cathet Cardiovasc Diagn 1996;39:36–42. [DOI] [PubMed] [Google Scholar]
- 14.Morrow WR, Palmaz JC, Tio FO, et al. Re-expansion of balloon-expandable stents after growth. J Am Coll Cardiol 1993;22:2007–13. [DOI] [PubMed] [Google Scholar]
- 15.Grifka RG, Vick GW, O’Laughlin MP, et al. Balloon expandable intravascular stents: aortic implantation and late further dilation in growing minipigs. Am Heart J 1993;126:979–84. [DOI] [PubMed] [Google Scholar]
- 16.Morrow WR, Smith VC, Ehler WJ, et al. Balloon angioplasty with stent implantation in experimental coarctation of the aorta. Circulation 1994;89:2677–83. [DOI] [PubMed] [Google Scholar]
- 17.Mendelsohn AM, Dorostkar PC, Moorehead CP, et al. Stent redilation in canine models of congenital heart disease: pulmonary artery stenosis and coarctation of the aorta. Cathet Cardiovasc Diagn 1996;38:430–40. [DOI] [PubMed] [Google Scholar]
- 18.Trerotola SO, Lund GB, Newman J, et al. Repeat dilation of Palmaz stents in pulmonary arteries: study of safety and effectiveness in a growing animal model. J Vasc Intervent Radiol 1994;5:425–32. [DOI] [PubMed] [Google Scholar]
- 19.McMahon CJ, El-Said HG, Grifka RG, et al. Redilation of endovascular stents in congenital heart disease: factors implicated in the development of restenosis and neointimal proliferation. J Am Coll Cardiol 2001;38:521–6. [DOI] [PubMed] [Google Scholar]
- 20.Spadoni I, Giusti S, Bertolaccini P. Long term follow-up of stents implanted to relieve peripheral pulmonary artery stenosis: haemodynamic findings and results of lung perfusion scanning. Cardiol Young 1999;9:585–91. [DOI] [PubMed] [Google Scholar]
- 21.Hijazi ZM, Al-Fadley F, Geggel RL, et al. Stent implantation for relief of pulmonary artery stenosis: immediate and short-term results. Cathet Cardiovasc Diagn 1996;38:16–23. [DOI] [PubMed] [Google Scholar]
- 22.Kirklin JW, Barratt-Boyes BG. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indications, 2nd ed. Edinburgh: Churchill Livingstone, 1993.
- 23.Aronberg DJ, Glazer HS, Madsen K, et al. Normal thoracic aortic diameters by computed tomography. Comput Assist Tomogr 1984;8:247–50. [PubMed] [Google Scholar]