Two major randomised trials concerning the surgical treatment of carotid artery stenosis showed that carotid endarterectomy is superior to medical treatment in reducing the overall risk of stroke in symptomatic or asymptomatic patients.w1–4 The first phase of the North American endarterectomy trial (NASCET)w1 confirmed the superiority for symptomatic severe (lumen diameter reduction > 70%) carotid stenoses while the second phase of the NASCET trial showed the benefit of carotid endarterectomy in patients with symptomatic moderate stenosis (50–70%).w4 The asymptomatic carotid study (ACAS) showed a reduction in stroke rate after carotid endarterectomy also in asymptomatic patients with carotid stenoses > 60%.w2
Although carotid endarterectomy is nowadays considered the gold standard for the treatment of carotid occlusive disease, the approach is not without complications. In the NASCET study population, 5.8% of patients suffered from perioperative death and stroke. When the attention is focused on routine clinical practice without strict patient selection criteria, or if a neurological audit is performed by independent neurologists, the incidence of major events can easily double.1
CAROTID STENTING: THE TIME OF THE PIONEERS
Since the first percutaneous transluminal carotid angioplasty was performed by Kerber in 1980,w5 the rapid improvement in interventional technology and materials has transformed a technique initially developed as a palliative treatment in inoperable patients into a therapeutic alternative option to surgery.w6–9 The main concern has always been the safety of such intervention in terms of cerebral embolisation, a frequent event during the procedure detected by transcranial Doppler monitoring, diffusion weighted magnetic resonance, and retinal fluoangioscopy even in asymptomatic patients.w10 w11 This may be explained by the presence of thrombotic material in carotid plaques frequently observed during surgical exploration.w12 Preliminary studies evaluating the safety and efficacy of carotid angioplasty showed a higher incidence of stroke and death compared to carotid endarterectomy.w7 w13–15 One randomised trial, comparing carotid angioplasty with carotid endarterectomy for symptomatic severe internal carotid artery disease, was aborted after enrolling only 17 patients because of the unacceptably high stroke/death rate following angioplasty (71%).w16 It is important, however, to point out that those patients were treated by interventionists with still limited carotid artery stenting experience, often using balloon expandable stents, at risk of late external compression, and were treated with inadequate antiplatelet therapy by today’s standards.
Increasing operator experience has led to better results, as reported in the recently published carotid and vertebral artery transluminal angioplasty study (CAVATAS) which randomised 504 patients with symptomatic carotid stenosis to either balloon angioplasty (bailout stenting was performed in 26%) or carotid endarterectomy. The results at 30 day and three year follow up were similar with both strategies (incidence of any stroke or any neurological deficit lasting more than seven days or death was 10% at 30 days).w17 Nevertheless, these results belong to the pre-stent era and cannot be applied to current treatment which considers stent implantation the main strategy to optimise percutaneous treatment of carotid artery stenosis.
While the main goal of carotid artery stenting is a more efficient resolution of the stenosis than is possible with balloons alone, it also aims to contain the atherosclerotic plaque within the stent barrier, promoting the formation of a smooth fibrotic neointima with low risk of distal embolisation and flow impairment. In 1998 one of the largest early series of carotid artery stenting, from 24 centres enrolling 2048 patients, reported a technical success rate of 98.6% with a combined periprocedural stroke and death rate of 5.77% (this rate varied from 0–10% in various centres).w18 Two years later the registry was enlarged to 4757 patients (carotid artery stent placement—CASP registry) and the combined periprocedural stroke and death rate reported was 5.07%.2 The most reliable data in terms of prospective single centre acquisition, independent pre- and postprocedure neurologic examination, and completeness of clinical and Doppler–angiographic follow up is offered by the series of Roubin and colleagues. Of the 528 consecutive patients treated between 1994 and 1999, before neuroprotection devices became available, only 17% would have qualified according to the inclusion criteria of the NASCET trial, indicating that the vast majority of patients were at high risk for surgery or were inoperable. Technical success was achieved in 98% of cases with an incidence of death and non-fatal major and minor strokes at 30 days of 1.6%, 1%, and 4.8%, respectively. A trend towards a progressive reduction of the rate of strokes (from 7.1% to 3.1% between 1994 and 1999) was observed as a result of the increased operators’ skills and availability of miniaturised dedicated catheters. Still, even including the learning phase, the overall incidence of a composite end point of death and stroke at 30 days of 7.4% compares favourably with the surgical results, especially when the high risk characteristics of the patients treated are considered. Imaging studies, available in more than 90% of patients and often including repeat angiography, showed a restenosis rate at six months of approximately 6%. The fear of late stent thrombosis or recurrence of embolisation is not supported by the long term follow up, which evaluated the incidence of major adverse neurological events for three years after the index procedure, with a rate of freedom from any ipsilateral stroke of 92%.3
Carotid artery stenting: technique overview.
Full documentation of the status of the two carotids and the vertebral arteries must be available before starting the procedure
A flexible sheath (6–7 French) or, more rarely, a guiding catheter (8–9 French) is advanced into the common carotid artery
The lesion is crossed and an occlusive balloon or a filter is positioned
Predilatation is performed only if it is felt that the filter or the stent cannot be advanced without it
A dedicated self expanding stent is selected, taking into consideration the need for 1–2 mm oversize of the unstretched diameter compared to the common carotid artery diameter. The stent is normally positioned across the origin of the external carotid artery, leaving liberal margins to cover the lesion proximally and distally
Postdilatation is performed after pretreatment with atropine using a 5–6 mm balloon
Final angiography is performed. Aspirin and clopidogrel are continued for one month
THE MOST IMPORTANT DEVELOPMENT AFTER THE STENT: NEUROPROTECTION
Ex vivo human carotid artery stenting models showed that embolic particles consist of atherosclerotic debris, organised thrombus, and calcified material.w12 The embolic potential of atherosclerotic plaque can be clinically assessed by ultrasound evaluation. Echolucent plaques are more frequently associated with emboli generation compared to echogenic plaques.w12 Furthermore, stenosis severity (> 90%), total length of the stenosis or the presence of multiple stenosis also significantly correlate with the total number of particles produced during the procedure and, consequently, with clinical outcome.4
Reimers and colleagues utilised three different types of distal protection devices in 88 consecutive lesions (84 patients) in internal carotid arteries that had > 70% diameter stenosis. Importantly, in 53% of filters, there was macroscopic evidence of debris.5 Collected material consisted of lipid-rich macrophages, fibrin material, and cholesterol clefts.6 A higher incidence of atheromatous debris (83%) was described by Tubler and colleagues7 who correlated the size of captured particles and the incidence of periprocedural neurological complications in 54 patients that underwent 58 carotid artery stenting procedures using distal balloon protection. Although there was a large overlap in the distribution of the particle size between patients with and without neurological complications, the authors concluded that the maximum area of aspirated particles is an indicator of increased risk for peri-procedural neurological complications.
Prompted by the observation of a high incidence of distal embolisation during carotid artery stenting, a variety of protection systems were designed to capture and remove atheromatous debris released during percutaneous interventions in carotid arteries. These systems can be divided into two major groups: balloon occlusive devices, and filter devices. Balloon occlusive devices can be further divided into proximal and distal devices according to the segment of carotid artery they occlude.
With all balloon occlusion devices, a major limitation concerns the potential to induce acute ischaemia of the homolateral cerebral hemisphere once the balloon is inflated, a situation that occurs in case of insufficient collateral circulation provided by the contralateral system.
The PercuSurge GuardWire system (Medtronic Inc, Santa Rosa, California, USA) combines a low profile high compliance balloon, to be inflated distal to the lesion, with a debris retrieval and aspiration system. Al-Mubarak and colleagues8 determined the effect of this device on the frequency of Doppler detected microembolic signals during carotid artery stenting in patients with (n = 37) and without (n = 39) distal protection. In patients without protection microembolic signals were observed during stent deployment, predilatation, and postdilatation. In those patients in whom the protection device was used, the frequency of microembolic signals was substantially reduced during these three phases. However, microembolic signals were still present in the protection group and detected predominantly during carotid sheath placement, guidewire manipulation, and distal balloon deflation.
Recently, Henry reported the results of carotid artery stenting using the PercuSurge GuardWire system in 184 lesions.9 Prophylactic occlusion during balloon dilation and stenting was well tolerated in 176 (95.7%) patients and technical success was 99.5%. Microscopic analysis of the aspirated blood showed different types of particles numbering between 7 and 145 per procedure, with a mean diameter of 250 μm (56–2652 μm). The 30 day stroke and death rate was 2.7% and the author concluded that protection devices may play an important role in future carotid interventions and expand the applicability of the procedure. Similar results are also reported by Schluter and colleagues in a consecutive series of 96 patients (102 lesions).10 The device was successfully delivered in 93 patients (97%) and temporary occlusion was tolerated in all but two patients (2.1%). In three patients the leakage of the GuardWire’s valve sealing ultimately ended in a non-protected procedure for a total feasibility rate of 92%. Major adverse neurologic events occurred in 3.1% of patients.
Randomised trials comparing surgery versus carotid artery stenting with and without cerebral protection are still ongoing and only limited results are available. The carotid angioplasty free of emboli (CAFE) pilot registry (Argentina, Germany, and USA) evaluated the first generation GuideWire protection system during carotid artery stenting. Whitlow and colleagues presented results from 40 patients enrolled and reported no strokes or deaths during a 30 day follow up period.11
However, the CAFE-USA trial, designed to assess feasibility, safety, and efficacy of the GuardWire Plus system in symptomatic (carotid stenosis > 60%) and asymptomatic (carotid stenosis > 70%) patients undergoing carotid Wallstent implantation, reported an incidence of death and stroke of 4.3% in the first 70 patients included in the registry.12
Contrary to the PercuSurge system, the clinical experience with the other balloon occlusive devices is limited. The Parodi Anti-Embolization System (PAES) (ArteriA, Inc, San Francisco, California, USA) is a large (11 French sheath/10 French protection system) triple lumen guiding catheter with an occlusion balloon attached to the outside of the catheter at the distal end. When a low pressure balloon is inserted to occlude the external carotid artery, the arterial sheath can be connected to the femoral vein creating an arterovenous fistula with flow reversal. The main advantage of this system is the ability to achieve cerebral protection without crossing the lesion, a dangerous source of embolisation in itself especially in a very irregular thrombus containing lesion or during emergency treatment of thrombotic occlusive lesions. In a small safety registry of 30 patients the proximal occlusive balloon had to be deflated in three of 30 patients because of acute cerebral ischaemia. Results of a larger registry with a system using active aspiration during balloon occlusion (MOMA) are expected to be released soon.
In contrast to the balloon based protection system, filters can prevent embolic events without interrupting blood flow distally and allowing continuous angiographic verification throughout the procedure. The main weaknesses of filters include the relatively large pore size (table 1) with the possibility of missing smaller particles, a relatively large crossing profile resulting in difficulties to cross tight or tortuous lesions, with the potential to cause spasm and dissection in the distal internal carotid artery (fig 1), and difficulties to retrieve the filter and withdraw it through the recently deployed stent. One of the major limitations in the use of filter based protection devices is severe carotid vessel tortuosity. In these settings, filter devices which are delivered over a guide wire already in position, such as the Trap filter (Microvena, White Bear Lake, Minnesota) or NeuroShield System (MedNova, Galway, Ireland), might have greater “pushability” and a better chance for a successful delivery/retrieval manoeuvre compared to those which are delivered simultaneously with the guide wire (AngioGuard, Cordis, Johnson & Johnson, Warren, New Jersey, USA; EPI Boston Scientific, Santa Clara, California, USA).
Table 1.
Features of different protection devices
| Device | Pore size (μm) | Crossing profile (inches) | Capture sheath profile (inches) | Diameters available (mm) |
| Angioguard XP | 100 | 0.042–52 | 0.066 | 4–8 |
| Mednova II | 120 | 0.058–68 | 0.096 | 4–6 |
| Mednova III | 140 | 0.046–51 | 0.084 | 4–6 |
| BSc FilterWire EZ | 80 | 0.039 | 0.039 | 3.5–5.5 |
| Medtronic AVE | 100 | 0.039 | 0.039 | 3.5–5.5 |
| Guidant Accunet | 120 | NA | NA | 4–8 |
| Microvena Trap | 200 | 0.037 | 0.066–78 | 2.5–7 |
| PercuSurge | No pores | 0.028–36 | 0.042–70 | 3–6 |
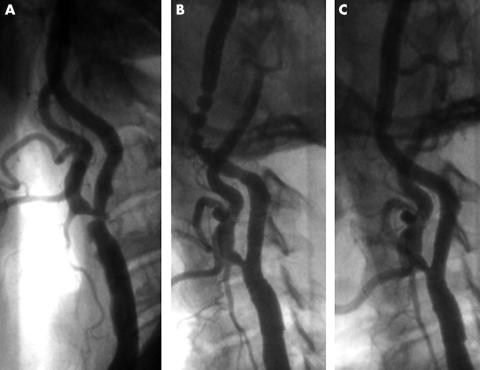
Figure 1.
Irregular ulcerated stenosis at the ostium of the left internal carotid artery in a patient experiencing repeated episodes of transient ischaemic attacks (panel A). After the delivery of a carotid Wallstent postexpanded with a 6.0 mm balloon a severe spasm is observed at the site of the MedNova filter device (panel B); the spasm is promptly resolved after filter retrieval (panel C).
CAROTID STENTING WITH NEUROPROTECTION: FROM EQUIVALENCY TO SUPERIORITY TO SURGERY
Clinical evaluation of carotid artery stenting performed under filter protection is still based on results obtained from small registries (table 29,10,12,13,15–17), while large randomised trials comparing carotid artery stenting with and without filter protection versus carotid endarterectomy are still ongoing.14 A recent multicentre registry reported the results of carotid artery stenting with distal protection in 308 patients, with 320 lesions in the internal carotid artery that had > 70% diameter stenosis. Three different devices were used: filters (80.6%), occlusive distal balloon (17.2%), and clamping of the common and external carotid arteries (2.2%). The procedural success rate was 95%. Thirty day major adverse neurological events rate was 2.5%.15
Table 2.
Major clinical experience of carotid artery stenting with distal protection
| Device | Patient/lesion (%) | Symptomatic (%) | Feasibility (%) | 30 day MAE (%) | Reference |
| PercuSurge | 167/184 | 50 | 95.7 | 2.7 | 9 |
| PercuSurge | 70 | 56 | 100 | 4.3 | 12 |
| PercuSurge | 96/102 | 46 | 92 | 3.1 | 10 |
| Parodi | 30 | 50 | 90 | 0 | 13 |
| Multiple devices | 308/320 | 59 | 95 | 2.5 | 15 |
| Mednova | 162/164 | 48 | 95 | 2 | 16 |
| Mednova | 50 | 42 | 98 | 4 | 17 |
| AngioGuard | 156 | 31 | 98 | 5.8 | * |
| AngioGuard | 408 | 42 | 98 | 6.9 | * |
*Yadav J, late breaking clinical trials, American Heart Association annual meeting 2002.
MAE, major adverse event.
A single centre experience reported the results of carotid artery stenting in 162 patients (164 lesions) using the Mednova NeuroShield filter device.16 Angiographic success was obtained in 99% of the lesions and the filter could be positioned in 95% of them. Thirty day event rate was 2% (two minor strokes and two deaths). The same type of filter device was also evaluated in a series of 50 consecutive patients (42 symptomatic) with internal carotid artery stenosis > 70%.17 Procedural success was 100% for stenting and 98% for filter placement/retrieval. Nevertheless, the death and major disability from stroke rate was 4% (two patients).
A multicentre randomised trial compared carotid stenting with distal protection to endarterectomy in high surgical risk patients (SAPPHIRE), defined as the presence of at least one of the following criteria: age > 80 years, congestive heart failure, obstructive lung disease, previous carotid endarterectomy, previous neck radiation or surgery, lesions distal or proximal to bifurcation. Clinical outcome was the incidence of major adverse events defined as death–stroke–myocardial infarction at 30 days and 30 day major adverse events plus death and ipsilateral stroke at 12 months. Non-randomised patients entered a registry either for carotid artery stenting (surgical refusal) or for carotid endarterectomy (interventional refusal). Carotid stenting was performed with the Nitinol carotid artery stent PRECISE and the protection device used was the Angioguard XP (both from Cordis, Miami, USA). Inclusion criteria were: common carotid artery or internal carotid artery stenosis > 50% in symptomatic or > 80% in asymptomatic patients, vessel diameter between 4–9 mm, and target lesion amenable to both carotid artery stenting and carotid endarterectomy. Results were recently presented (Yadav J, late breaking clinical trials, American Heart Association annual meeting 2002). The patients were randomised to carotid artery stenting (n = 156) and carotid endarterectomy (n = 151). Among the stented patients, procedure success—defined as residual diameter stenosis < 30%—was achieved in 91% of lesions, and AngioGuard delivery/retrieval success was 98%. The incidence of 30 day major adverse events was 5.8% in the carotid artery stenting arm and 12.6% in the carotid endarterectomy arm (p = 0.047). The difference in favour of carotid artery stenting was large both in symptomatic (4.2% v 15.4%) and asymptomatic (6.7% v 11.2%) patients. During the study period, 416 patients could not enter the trial because they were felt to be at too high a risk or unsuitable for carotid endarterectomy (409 patients). The results obtained in these patients with carotid stenting were similar to those of the patients randomised to carotid artery stenting (30 day major adverse event rate 7.8%). The high rate of patients refused by surgeons confirms the wider applicability of carotid stenting, which was felt to be non-applicable in only seven of 717 patients screened. Finally, cranial nerve injury was present in 5.3% of carotid endarterectomy patients but in none of the carotid artery stenting patients (p < 0.01).
Carotid artery stenting: key points.
Randomised trials of surgical coronary endarterectomy have demonstrated that medical treatment is inferior to carotid endarterectomy in symptomatic patients with > 50% stenosis and asymptomatic patients with > 70% stenosis
Carotid artery stenting can potentially offer the same advantage as surgery by excluding the friable plaque material, enlarging the lumen, and promoting the formation of a thin fibrotic neointima
The randomised CAVATAS trial and the well controlled consecutive series of Roubin and colleagues demonstrate that endovascular techniques can be applied in patients who are poor surgical candidates or inoperable and have an incidence of mortality and major and minor stroke comparable to the incidence observed with surgical carotid endarterectomy
Unlike coronary plaques, carotid plaques are very friable, contain thrombus, and are at high risk of distal embolisation, which is detectable in almost all cases when transcranial Doppler is performed or the particulate is collected with the use of distal protection devices
Neuroprotection is mandatory and is performed with the use of an occlusion balloon or, more frequently, with the use of filters to maintain flow during treatment
The low percentage of minor and major strokes observed in large series of consecutive patients treated with neuroprotection is in agreement with the lower event rate reported in the only randomised trial of carotid stenting with distal filters versus carotid endarterectomy
The Acculink for revascularization of carotids in high risk patients (ARCHER) study will also evaluate the safety and efficacy of the Acculink carotid stent system in patients at high risk or unsuitable for carotid endarterectomy (planned enrolment 398 patients).
CONCLUSIONS
The results of large randomised trials must be awaited in order to confirm the data gathered from large registries and one small scale randomised study which suggest a lower incidence of periprocedural infarction and stroke with carotid artery stenting with distal protection versus carotid endarterectomy. Carotid artery stenting is also a safe and effective alternative to surgical carotid endarterectomy for treatment of carotid occlusive disease in patients who are inoperable or at high surgical risk. As the procedure is also less invasive and better tolerated, a rapid shift from carotid endarterectomy to carotid stenting is expected.
Supplementary Material
REFERENCES
- 1.Chaturvedi S, Aggarwal R, Murugappan A. Results of carotid endarterectomy with prospective neurologist follow-up. Neurology 2000;55:769–72. [DOI] [PubMed] [Google Scholar]
- 2.Wholey MH, Wholey M, Mathias K, et al. Global experience in cervical carotid artery stent placement. Catheter Cardiovasc Interv 2000;50:160–7. [DOI] [PubMed] [Google Scholar]
- 3.Roubin GS, New G, Iyer SS, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis: a 5-year prospective analysis. Circulation 2001;103:532–7. ▸ In the largest prospective cohort of patients (528 patients, 604 arteries) conducted with neurological independent audits, the mortality rate was 1.6%, non-fatal major stroke 1%, and minor stroke 4.8% (overall 30 day stroke and death rate 7.4%). [DOI] [PubMed] [Google Scholar]
- 4.Mathur A, Roubin GS, Iyer SS, et al. Predictors of stroke complicating carotid artery stenting. Circulation 1998;97:1239–45. ▸ Multivariate analysis revealed that the predictors of procedural stroke in 231 consecutive patients undergoing carotid stenting were advanced age and presence of lung or multiple lesions. [DOI] [PubMed] [Google Scholar]
- 5.Reimers B, Corvaja N, Moshiri S, et al. Cerebral protection with filter devices during carotid artery stenting. Circulation 2001;104:12–5. ▸ This first consecutive multicentre series of the use of distal filter protection devices in 88 consecutive lesions showed a successful positioning in 96.5% of cases, with retrieval of macroscopic evidence of debris in 53%. Only one neurological complication (minor stroke 1.2%) was observed. [DOI] [PubMed] [Google Scholar]
- 6.Angelini A, Reimers B, Della Barbera M, et al. Cerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debris. Stroke 2002;33:456–61. [DOI] [PubMed] [Google Scholar]
- 7.Tubler T, Schluter M, Dirsch O, et al. Balloon-protected carotid artery stenting: relationship of periprocedural neurological complications with the size of particulate debris. Circulation 2001;104:2791–6. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mubarak N, Roubin GS, Vitek JJ, et al. Effect of the distal-balloon protection system on microembolization during carotid stenting. Circulation 2001;104:1999–2002. [DOI] [PubMed] [Google Scholar]
- 9.Henry M, Henry I, Klonaris C, et al. Benefits of cerebral protection during carotid stenting with the PercuSurge GuardWire system: midterm results. J Endovasc Ther 2002;9:1–13. [DOI] [PubMed] [Google Scholar]
- 10.Schluter M, Tubler T, Mathey DG, et al. Feasibility and efficacy of balloon-based neuroprotection during carotid artery stenting in a single-center setting. J Am Coll Cardiol 2002;40:890–5. ▸ Large consecutive single centre series of 102 carotid stenting procedures protected with the use of a distal occlusion balloon (PercuSurge). With 92% procedural feasibility, three strokes and two transient ischaemic attacks occurred, two in patients where the device prematurely deflated because of poor subjective tolerance. [DOI] [PubMed] [Google Scholar]
- 11.Whitlow P, Lylyk P, Londero H, et al. Protected carotid stenting with the PercuSurge Guardwire: results from a multi specialty study group [abstract]. J Am Coll Cardiol 2000;35:85. [Google Scholar]
- 12.Roubin G, Mehran R, Diethrich E, et al. Carotid stent-supported angioplasty with distal neuro-protection using the GuardwireTM: 30-day results from the carotid angioplasty free of emboli (CAFÉ-USA) trial [abstract]. J Am Coll Cardiol 2001;37:1A–648A.11153722 [Google Scholar]
- 13.Adami CA, Scuro A, Spinamano, et al. Use of the Parodi anti-embolism system in carotid stenting: Italian trial results. J Endovasc Ther 2002;9:147–54. [DOI] [PubMed] [Google Scholar]
- 14.Roubin GS, Hobson RW, 2nd, White R, et al. CREST and CARESS to evaluate carotid stenting: time to get to work! J Endovasc Ther 2001;8:107–10. [DOI] [PubMed] [Google Scholar]
- 15.Reimers B, Castriota F, Corvaja N, et al. Carotid artery stent implantation with cerebral protection: a multicenter experience of 320 procedures [abstract]. J Am Coll Cardiol 2001;39:812–30A. [Google Scholar]
- 16.Al-Mubarak N, Colombo A, Gaines PA, et al. Multicenter evaluation of carotid artery stenting with a filter protection system. J Am Coll Cardiol 2002;39:841–6. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald S, Venables GS, Cleveland TJ, et al. Protected carotid stenting: safety and efficacy of the MedNova NeuroShield filter. J Vasc Surg 2002;35:966–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.