Abstract
Objectives: To define the ability of early exercise testing and coronary angiography to predict prognosis in young survivors of myocardial infarction (MI).
Methods: 255 consecutive patients (210 men) aged 55 years or less (mean 48 years) admitted to hospital (1981–85) were eligible. Of these, 150 patients (130 men) who were able to exercise early after MI and underwent coronary angiography within six months constituted the study group and were followed up for up to 15 years. Survival data up to 18 years was obtained for the whole cohort.
Results: Survival at a median of 16 years was 52% for the whole cohort, 62% for the study group, and 48% for the excluded group. From nine years onwards survival deteriorated significantly in the study group compared with an age matched background population. Fifteen years after MI, 121 patients (81%) in the study group had had at least one event (death, MI, revascularisation, cardiac readmission, stroke) leaving 29 (19%) event-free. The number of diseased vessels was the major determinant of time to first event (p = 0.001) and event-free survival (p = 0.04). Exercise duration was also important in the prediction of time to first event (p = 0.003). Death was influenced by a history of prior MI.
Conclusion: The favourable initial survival was followed by significant deterioration after nine years. This late attrition is an important treatment target. Furthermore, this study supports risk stratification early after MI combining angiography with non-invasive tools.
Keywords: long term survival, young, myocardial infarction, coronary angiography
Survival after myocardial infarction (MI) is influenced by multiple factors, of which age stands out as a major non-modifiable predictor of long term prognosis.1 Although short and medium term prognosis in young MI survivors is known to be excellent,2–4 little is known about their long term prognosis. This prospective study was carried out over 18 years and investigated the factors that influence the survival of patients with MI stratified by early exercise testing, early angiography, and clinical parameters.
METHODS
Patients
A total of 255 consecutive patients (210 men) aged 55 years or less with mean age of 48 years were admitted to the coronary care unit of the Royal Victoria Infirmary with an MI between October 1981 and March 1985. The inhospital and three month mortality accounted for 24 (9%) deaths. Of the remaining cohort, 150 subjects (130 men) who were able to exercise after their MI and who agreed to have coronary angiography within six months of enrolment constituted the study group.
Excluded group
The remaining 81 who either did not qualify or did not consent to early exercise testing or angiography were excluded. Of these, 37 had cardiac and non-cardiac contraindications to exercise testing (13 and 24, respectively), 14 refused to consent, and 30 were excluded because either the angiogram or the exercise test was not performed at the correct time. Except for nine (6%), none of the enrolled patients received thrombolysis. All excluded patients were also traced and information on their survival status was obtained by using the Office for National Statistics (ONS) central register.
Diagnosis of acute infarction
The diagnosis of acute infarction was based on at least two of the following: firstly, a history of typical chest pain lasting more than 30 minutes; secondly, characteristic ECG changes of acute MI; and thirdly, increase of the cardiac enzyme creatine kinase to at least twice the normal upper limit.
Study group population
One hundred fifty subjects who were able to exercise early after the MI and consented to undergo coronary angiography constituted the study group population.
A maximal exercise test was performed according to the modified Bruce protocol six weeks after the MI. Criteria of a positive exercise test have been published previously.3
A coronary angiogram and left ventriculography were performed at a mean of 3 (range 1–6) months’ after MI and reviewed by two independent observers. Stenoses (visualised in at least two orthogonal planes) of greater than 50% intraluminal diameter reduction were considered significant.
Follow up
The study group cohort was followed up at 1.5, 3, 5, 7, 9, 12, and 15 years. These assessments included formal clinical review as outpatients, completion of questionnaires, and tracing of hospital records. In addition we contacted the treating physicians of patients who had moved to other areas. Deaths, MI, revascularisation (coronary artery bypass surgery or percutaneous transluminal coronary angioplasty), readmission for cardiac reasons, repeat angiography, and stroke were defined prospectively and recorded as “events”. Revascularisation was performed only for clinical indications and not for angiographic findings alone. Information on survival for the whole cohort was obtained by prospectively registering all patients with the ONS central register. This means that we would have been notified of all deaths occurring in the UK. Only 4.6% (7) of patients from the study group and 19% (20) from the excluded group were lost from follow up 18 years after the index infarction.
Background population
Tables showing the probability of survival among the general population for subjects of ages and sex comparable with those in our population were constructed from data extrapolated from 1982–84 life tables prepared by the government’s actuary department.
Statistical analysis
Statistical analysis was performed using the STATA (version 6) software package. Following univariate testing a multivariate survival analysis using the stepwise Cox proportional hazards method and logistic regression were applied to assess predictors of long term prognosis. The Cox proportional hazard method models the event times and the stepwise technique was used to choose predictor variables from a large set of other variables. The overall survival analyses were determined by the Kaplan-Meier method. The χ2 test was used for analysis of variables where logistic regression was not appropriate and to test differences in survival compared with the background population. Differences in survival between the study and excluded groups were assessed with a log rank test. Survival calculations based on age specific mortality rates were used to construct life expectancy tables. The analysis deals with three response variables—namely, the time to first event, time to death, and event-free survival. Variables examined for their predictive value were sex, site of infarction, Q wave or non-Q wave infarction, exercise test variables, smoking, prestudy infarction, ejection fraction, the number of diseased vessels, and the site of stenoses. Probability values of p ≤ 0.05 were considered significant.
RESULTS
Clinical characteristics of the study group
In the study group of 150 patients, a Q wave infarction was found in 115 (77%) and 62 (41%) of the infarcts were anterior. Left ventricular ejection fraction was ≤ 40% in 26 (18%). Angiography showed left main stem disease in 3 (2%), three vessel disease in 24 (16%), two vessel disease in 53 (35%), one vessel disease in 65 (43%), and no significant coronary disease in 5 (3%).
Follow up
The follow up data consists of the clinical follow up, which was at 15 years following infarction for the study group (section A), and data for survival in the whole cohort up to 18 years after the beginning of the study (median 16 years) from the ONS (section B).
Section A: outcome of the study group at 15 years after MI
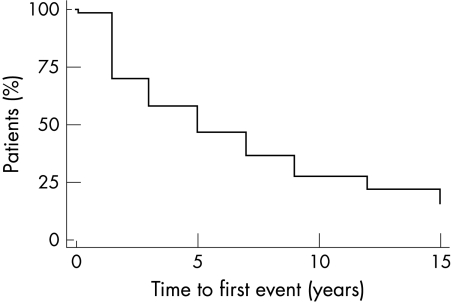
During follow up 44 (29%) patients died and 121 (81%) had at least one event (100% of women and 78% of men) (table 1). Thus, there were only 29 (19%) subjects with event-free survival. Revascularisation was performed in 58 (39%), 40 (27%) suffered reinfarction, 26 (17%) had a stroke, 40 (27%) underwent repeat angiography, and 71 (47%) were readmitted for cardiac related events. Table 1 also shows results of outcome in relation to exercise variables, angiographic findings, and other baseline characteristics such as history of prior MI. Events were most frequent during the first 18 months of follow up, during which 31% of survivors experienced an event (fig 1).
Table 1.
Long term outcome in the study group: baseline characteristics, coronary anatomy, and six week exercise variables in relation to events 15 years after the index infarction
| CABG/PTCA (n=58) | MI (n=40) | Death (n=44) | Re-angiography (n=40) | Stroke (n=26) | Event-free (n=29) | |
| Men | 51 (39%) | 35 (27%) | 37 (25%) | 38 (29%) | 19 (15%) | 29 (22%) |
| Previous MI | 3 (60%) | 2 (40%) | 3 (60%) | 1 (20%) | 3 (60%) | 0 |
| Smoking history | 48 (38%) | 35 (28%) | 39 (31%) | 33 (26%) | 23 (18%) | 23 (18%) |
| Q wave MI | 45 (39%) | 30 (26%) | 34 (30%) | 30 (26%) | 16 (14%) | 23 (20%) |
| Anterior MI | 19 (31%) | 13 (21%) | 23 (37%) | 9 (15%) | 9 (15%) | 11 (18%) |
| Treadmill exercise test (ETT) | ||||||
| Duration ≥15 minutes | 26 (30%) | 23 (27%) | 20 (23%) | 27 (31%) | 12 (14%) | 23 (27%) |
| ST depression | 11 (42%) | 8 (31%) | 3 (12%) | 8 (31%) | 5 (19%) | 5 (19%) |
| Angina | 27 (57%) | 11 (23%) | 14 (30%) | 11 (23%) | 7 (15%) | 6 (13%) |
| Angiographic findings | ||||||
| LVEF ≤40% | 10 (38%) | 5 (19%) | 11 (42%) | 5 (19%) | 1 (4%) | 3 (12%) |
| Normal coronaries | 0 | 1 (20%) | 0 | 0 | 1 (20%) | 3 (60%) |
| 1VD | 16 (25%) | 18 (28%) | 21 (32%) | 11 (17%) | 7 (11%) | 17 (26%) |
| 2VD | 21 (40%) | 13 (25%) | 13 (25%) | 19 (36%) | 11 (21%) | 7 (13%) |
| 3VD | 19 (79%) | 6 (25%) | 8 (33%) | 8 (33%) | 6 (26%) | 1 (4%) |
| Left main stem disease | 3 (100%) | 2 (67%) | 2 (67%) | 1 (33%) | 1 (33%) | 0 |
CABG, coronary artery bypass grafting; ETT, exercise tolerance test six weeks after infarction; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; VD, vessel disease.
Figure 1.
Kaplan-Meier plot of the time to first event in the study group.
Time to first event and event-free survival: multivariate analysis
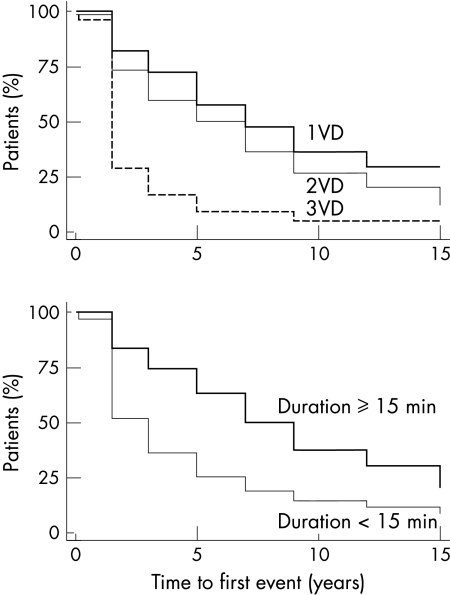
The number of diseased vessels significantly influenced the time to first event (p = 0.001) (tables 2 and 3). The more diseased vessels the higher the hazard of an event (fig 2). The hazard ratio (compared with one vessel disease) increased with the number of vessels diseased—that is, it was 1.31 (95% confidence interval (CI) 0.86 to 2.00) for two vessel disease and 2.70 (95% CI 1.60 to 4.57) for three vessel disease. Similarly, the influence of the number of diseased vessels was related to event-free survival (p = 0.04), with an event odds ratio of 2.62 (95% CI 0.97 to 7.10) for two vessel disease and 8.35 (95% CI 1.03 to 67.7) for three vessel disease when compared with one vessel disease.
Table 2.
Univariate analysis of the time to first event at 15 years after MI
| Variable | Hazard ratio (95% CI) | p Value |
| 1VD v 2VD | 1.33 (0.88 to 2.04) | <0.001 |
| 1VD v 3VD | 3.02 (1.79 to 5.09) | |
| ETT duration ≥15 minutes | 0.50 (0.34 to 0.71) | <0.001 |
| Previous MI | 2.96 (1.20 to 7.34) | 0.02 |
| Angina (ETT) | 1.51 (1.03 to 2.21) | 0.03 |
| Men | 0.61 (0.38 to 0.99) | 0.05 |
| Q wave MI | 0.99 (0.65 to 1.50) | 0.95 |
| Anterior MI | 1.09 (0.76 to 1.57) | 0.64 |
| LAD/LMS disease | 1.32 (0.88 to 1.97) | 0.17 |
| EF ≤40% | 1.5 (0.94 to 2.31) | 0.08 |
| ST depression (ETT) | 0.92 (0.57 to 1.47) | 0.73 |
| Smoking | 1.15 (0.69 to 1.91) | 0.60 |
| Smoking cessation | 0.87 (0.59 to 1.28) | 0.48 |
CI, confidence Interval; EF, ejection fraction; LAD, left anterior descending coronary artery; LMS, left main stem.
Table 3.
Results of stepwise Cox multivariate analysis showing significant predictors 15 years after MI in the study group
| Variable | p Value | Hazard ratio (95% CI) | Odds ratio (95% CI) |
| Time to first event | |||
| Number of diseased vessels | |||
| 1VD v 2VD | 0.001 | 1.31 (0.86 to 2.00) | NA |
| 1VD v 3VD | 2.70 (1.60 to 4.57) | NA | |
| ETT duration ≥15 mins | 0.003 | 0.56 (0.38 to 0.82) | NA |
| Event-free survival | |||
| Number of diseased vessels | |||
| 1VD v 2VD | 0.04 | NA | 2.62 (0.97 to 7.10) |
| 1VD v 3VD | NA | 8.35 (1.03 to 67.7) | |
| ETT duration ≥15 mins | 0.05 | NA | 0.35 (0.13 to 0.98) |
| Time to death | |||
| Previous MI | 0.04 | 3.39 (1.04 to 11.02) | NA |
| ST depression (ETT) | 0.04 | 0.28 (0.09 to 0.92) | NA |
NA, Not applicable.
Figure 2.
Kaplan-Meier estimate of the time to first event in relation to the number of diseased vessels (upper panel) and duration of exercise test at six weeks after infarction (lower panel) in the study group. VD, vessel disease.
Figure 2 shows the effect of exercise duration on time to first event (hazard ratio 0.56, 95% CI 0.38 to 0.82, p = 0.003) and event-free survival (odds ratio 0.35, 95% CI 0.13 to 0.98, p = 0.05). Patients who had an exercise capacity of ≥ 15 minutes had less hazard of suffering future events than did patients who exercised for less than 15 minutes on the treadmill.
Time to death
Death was influenced significantly both by a history of MI before the index infarction and by ST depression on exercise testing (p = 0.04) with a hazard ratio of 3.39 (95% CI 1.04 to 11.02) and 0.28 (95% CI 0.09 to 0.92), respectively (table 3). Angiographic ejection fraction at a cut off point of 40% did not significantly effect the time to death (p = 0.3).
Section B: mortality at up to 18 years in the whole cohort of 255 patients
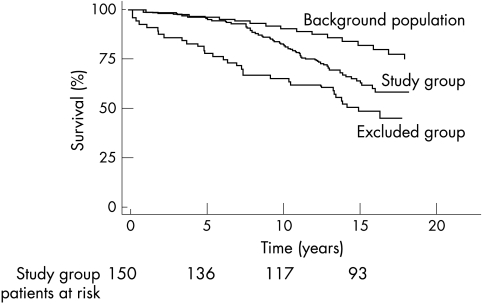
There were 110 deaths (48%) in the whole cohort (27 lost to follow up) after a median interval of 16 years. These data were obtained by prospectively registering the patients with the ONS. At this time the death rate of the excluded group was 52% compared with 38% in the study group (fig 3). The difference in mortality between the study and excluded groups became significant at seven years and was maintained up to 18 years (p = 0.03). Compared with the background population, in the study group mortality was significantly higher from nine years onwards (p < 0.001). Mode of death was not significantly different between study and excluded groups (p = 0.12).
Figure 3.
Survival up to 18 years (median 16 years) after myocardial infarction in the study group, excluded group, and background population.
DISCUSSION
Long term survival
This is the longest follow up study of its kind. It is the first report on long term outcome of young MI sufferers and the predictive value of early exercise testing and coronary angiography within six months of infarction. Up until eight years after the MI the survival rate was excellent among young MI survivors who were able to perform an exercise test after their MI with no significant difference from the age and sex matched background population. However, this favourable trend unequivocally deteriorated with advancing age as death rates accelerated with time. The survival curve of the excluded patients diverged from that of the background population immediately after the index infarction and continued to do so until the end of follow up. Thus, during the 14–18 year (median 16) observation period, 38% of patients who performed an exercise test and 52% of the excluded patients had died.
The impact of age on outcome has been addressed by many studies in different treatment eras. Generally, survival is regarded as favourable in young MI sufferers, and if we had followed up the study population for only eight years we would not have detected the later acceleration in death rate and our conclusions would have been different.
Patients recruited for this study suffered their MI in the prethrombolytic era and as yet it would not be possible to follow up a cohort of thrombolysed patients for a comparable period. Recent studies have shown that the early benefits of thrombolysis are sustained at 10 years,5–7 and this was most pronounced in those under 65 years of age. Thus, had our patients received thrombolysis we would expect a somewhat better survival and we do not know whether the late deterioration we observed would still have occurred. However, since late deterioration is likely to be related to advancing severity of coronary disease with age there is a strong possibility it would also be seen in thrombolysed patients. Thrombolysis, primary angioplasty, and other advances of contemporary management have had a major impact on the acute case fatality of MI.8 Since early mortality is low, future improvements in early care are unlikely to have a great impact on the survival rate of patients with MI. We have shown that the late attrition of such patients is considerable and presents an important treatment target. Understanding the determinants of late death are essential if this target is to be hit.
Early exercise testing
This study examined predictivity of exercise duration and number of diseased coronary arteries over a much longer follow up than previously achieved.9,10 Patients who exercised early after an MI for 15 minutes or more were half as likely to suffer an event as those who did less. Furthermore, a long exercise time predicted event-free survival. The value of early exercise testing in the long term risk assessment after MI is controversial.11,12 In recent years there has been a growing impression that exercise variables (mainly ST changes and angina) seem to lose their predictive ability from the medium term (five years) onwards.13,14 However, in this study, exercise capacity retained a predictive value for 15 years after an MI. We also found a seemingly paradoxical association between ST segment depression six weeks after MI and time to death, with patients who did not develop ST depression being at a higher risk of death than those who did. This finding was somewhat unexpected although supported by another publication where patients with negative exercise tests had the highest number of new MIs and the highest incidence of sudden coronary death.15 The explanation may be that ST depression represents viable myocardium, which can improve its function and contribute to overall left ventricular performance following either collateral development or revascularisation.
Coronary angiography
The severity of coronary artery disease was an extremely important predictor of outcome. Patients with three vessel disease had almost three times the hazard of suffering an event and were eight times less likely to have an event-free survival than patients with one vessel disease during 15 years of follow up. Although angiography is the most accurate way of defining anatomical disease severity, whether this should be considered for all MI survivors is still controversial. While being recommended by some researchers in conjunction with other selected non-invasive tests as a routine risk stratification tool after an MI,16 others have suggested that there was no benefit of routine angiography over a conservative approach.17 Our study with its long term findings supports the view that routine angiography after MI provides valuable clinical prognostic information. Although the length of our study is its strong point, in fact the effect of three vessel disease on events appears early. It may be argued that this was because three vessel disease led to coronary revascularisation being performed on purely angiographic grounds. This was not the case, as this confounding factor was recognised in the initial protocol, which was written at a time when the prognostic benefit of coronary artery bypass surgery in three vessel disease was not fully recognised. It was also decided that with the exception of left main stem disease (three patients) revascularisation would be performed only for symptoms. There is no convincing evidence that intervention (coronary artery bypass surgery or percutaneous transluminal coronary angioplasty) after MI improves survival, and results from studies that compared invasive versus conservative approach showed no significant difference between the two groups in terms of mortality.18 Such studies may, however, have been far too short term to show benefit, and our study should encourage longer follow up in postinfarct studies. The DANAMI (Danish multicentre randomised study of invasive versus conservative treatment in patients with inducible ischaemia after thrombolysis in acute myocardial infarction) showed significant benefit in morbidity but not in mortality except when death was considered for combined end point or time to first event. However, at the end of four years (median 2.4 years) of follow up the two curves continued to diverge and it is probable that the benefit in survival would manifest at a later stage of follow up.
Young MI survivors have less severe coronary disease than older patients,19,20 which may explain their early favourable outcome.
Left ventricular dysfunction would be expected to influence prognosis but this study and others14,21–23 suggest that this effect is most apparent in the medium (up to five years) rather than long term. This is probably because those patients who have severe left ventricular damage die in the early years of follow up.
Limitations and conclusion
Over the years new treatments for coronary artery disease have appeared. The most exciting recent developments have been evidence of survival benefit for the statins and angiotensin converting enzyme inhibitors.24,25 These agents in combination with other tried and tested treatments such as aspirin and β blockers26,27 will inevitably change long term outcome. Our study gives the first clear idea of the long term pattern of survival on which the benefits of these new modalities will be superimposed. This comparison will be weakened to some extent by the treatment of our cohort with these agents as these new developments have emerged. However, this study has shown that intense effort to stratify patients after infarction and to maintain careful follow up provide useful information. Perhaps late deaths will be reduced dramatically by modern medical treatment but our study also suggests that complete evaluation with angiography combined with the inexpensive exercise stress test allows those at risk to be better defined and revascularisation to be undertaken in a timely manner. Finally, those at very low risk can be identified and told of their good prognosis.
Acknowledgments
This study was supported by the British Heart Foundation (BHF) and Abu Dhabi Investment Authority (ADIA).
REFERENCES
- 1.Goldberg RJ, McCormick D, Gurwitz JH, et al. Age-related trends in short- and long-term survival after acute myocardial infarction: a 20-year population-based perspective (1975–1995). Am J Cardiol 1998;82:1311–7. [DOI] [PubMed] [Google Scholar]
- 2.Fulhaas JU, Rickenbacher P, Pfisterer M, et al. Long-term prognosis of young patients after myocardial infarction in the thrombolytic era. Clin Cardiol 1997;20:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peart I, Odemuyiwa O, Albers C, et al. Exercise testing soon after myocardial infarction: its relation to course and outcome at one year in patients aged less than 55 years. Br Heart J 1989;61:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odemuyiwa O, Peart I, Albers C, et al. Exercise testing at 3 weeks, 6 weeks and 18 months after infarction and the outcome at 3 years in young patients (under 55 years). Eur Heart J 1992;13:936–41. [DOI] [PubMed] [Google Scholar]
- 5.Franzosi MG, Santoro E, De-Vita C, et al. Ten-year follow-up of the first megatrial testing thrombolytic therapy in patients with acute myocardial infarction: results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto-1 study. The GISSI investigators. Circulation 1998;98:2659–65. [DOI] [PubMed] [Google Scholar]
- 6.Baigent C, Collins R, Appleby P, et al. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second international study of infarct survival) collaborative group. BMJ 1998;316:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenderink T, Simmons ML, Van-Es GA, et al. Benefit of thrombolytic therapy is sustained throughout five years and is related to TIMI perfusion grade 3 but not grade 2 flow at discharge. The European Cooperative Study Group. Circulation 1995;92:1110–6. [DOI] [PubMed] [Google Scholar]
- 8.Levy D, Thom TJ. Death rates from coronary disease: progress and a puzzling paradox. N Eng J Med 1998;339:915–7. [DOI] [PubMed] [Google Scholar]
- 9.Ekstrand K, Bostrom PA, Lilja B, et al. Submaximal early exercise test compared to clinical findings for evaluation of short- and long-term prognosis after the first myocardial infarction. Result from the MONICA Project in Augsburg and Toulouse. Eur Heart J 1997;18:822–34. [DOI] [PubMed] [Google Scholar]
- 10.Weiner DA, Ryan TJ, Parsons L, et al. Long-term prognostic value of exercise testing in men and women from the coronary artery surgery study (CASS) registry. Am J Cardiol 1995;75:865–70. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson R, Umachandran V, Ranjadayalan K, et al. Reassessment of treadmill stress testing for risk stratification in patients with acute myocardial infarction treated by thrombolysis. Br Heart J 1993;70:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pridie RB. Has thrombolytic therapy changed the equation for post infarction risk stratification? Lancet 1997;349:1028. [DOI] [PubMed] [Google Scholar]
- 13.Casella G, Pavesi PC, Medda M, et al. Long-term prognosis of painless exercise-induced ischaemia in stable patients with previous myocardial infarction. Am Heart J 1998;136:894–904. [DOI] [PubMed] [Google Scholar]
- 14.Pepine CJ. Prognostic markers in thrombolytic therapy: looking beyond mortality. Am J Cardiol 1996;78:24–7. [DOI] [PubMed] [Google Scholar]
- 15.McHenry PL, O’Donell J, Morris SN, et al. The abnormal exercise electrocardiogram in apparently healthy men: a predictor of angina pectoris as an initial coronary event during long-term follow-up. Circulation 1984;70:547–51. [DOI] [PubMed] [Google Scholar]
- 16.Kulick DL, Rahimtoola SH. Risk stratification in survivors of acute myocardial infarction: routine cardiac catheterization and angiography is a reasonable approach in most patients. Am Heart J 1991;121:641–56. [DOI] [PubMed] [Google Scholar]
- 17.Peterson ED, Shaw LJ, Callif RM. Risk stratification after myocardial infarction. Ann Intern Med 1997;126:561–82. [DOI] [PubMed] [Google Scholar]
- 18.Madsen JK, Grande P, Saunamäki K, et al. Danish multicenter randomised study of invasive versus conservative treatment in patients with inducible ischaemia after thrombolysis in acute myocardial infarction (DANAMI). Circulation 1997;96:748–55. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman FH, Cameron A, Fisher LD, et al. Myocardial infarction in young adults: angiographic characterisation, risk factors and prognosis (coronary artery surgery study registry). J Am Coll Cardiol 1995;26:654–61 [DOI] [PubMed] [Google Scholar]
- 20.Garoufalis S, Kouvaras G, Vitsias G, et al. Comparison of angiographic findings, risk fsctors and long-term follow-up between young and old patients with a history of myocardial infarction. Int J Cardiol 1998;67:75–80. [DOI] [PubMed] [Google Scholar]
- 21.Olona M, Candell-Riera J, Permanyer-Miralda G, et al. Strategies for prognostic assessment of uncomplicated first myocardial infarction: 5 year follow-up study. J Am Coll Cardiol 1995;25:815–22. [DOI] [PubMed] [Google Scholar]
- 22.Volpi A, Cavalli A. High and low risk groups: early and late prognostic stratification. J Cardiovasc Risk 1994;1:295–300. [PubMed] [Google Scholar]
- 23.Skinner JS, Albers C, Goudevenos J, et al. Prospective study of patients aged 55 years or less with acute myocardial infarction between 1981 and 1985: outcome 7 years and beyond. Br Heart J 1995;74:606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anon. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- 25.Anon. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE investigators. N Engl J Med 1992;327;669–77. [DOI] [PubMed] [Google Scholar]
- 26.Collaborative overview of randomised trials of antiplatelet therapy I: prevention of death, myocardial infarction and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet trialists’ collaboration. BMJ 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 27.Cucherat M, Boissel JP, Leizorovicz A. Persistent reduction of mortality for five years after one year of acebutolol treatment initiated during acute myocardial infarction. The APSI investigators. Am J Cardiol 1997;79:587–9. [DOI] [PubMed] [Google Scholar]