Abstract
Background: Raman spectroscopy has the unique potential to detect and quantify cholesterol and calcification in an atherosclerotic plaque in vivo.
Objective: To evaluate the sensitivity and specificity of this technique for detecting cholesterol or calcification in human coronary artery and aorta specimens ex vivo, using a compact clinical fibreoptic based Raman system developed for in vivo applications.
Design: From nine coronary arteries and four aorta specimens, 114 sites were evaluated for the presence of cholesterol and calcification by Raman spectroscopy and standard histology. Raman spectra were acquired and evaluated on-line in around five seconds.
Results: The correlation between Raman spectroscopy and histology was r = 0.68 for cholesterol and r = 0.71 calcification in the plaque (p < 0.0001). Sensitivity and specificity for detecting cholesterol and calcification were excellent: receiver operating characteristic (ROC) analysis for each of the components revealed areas under the curves of > 0.92 (p < 0.0001). At the optimal cut-off values determined by ROC analysis, positive predictive values of > 80% and negative predictive values of > 90% were obtained.
Conclusions: On-line real time catheter based Raman spectroscopy detects accumulation of cholesterol and calcification in atherosclerotic plaque with high sensitivity and specificity.
Keywords: atherosclerosis, vulnerable plaque, Raman spectroscopy
Plaque vulnerability is related, in part, to its molecular composition. The risk of sudden rupture or fissure is largest in plaques characterised by high extracellular lipid content, abundant macrophages, small numbers of smooth muscle cells, and relatively low concentrations of collagen and glycosaminoglycans.1 If a rupture occurs, a small lesion may rapidly progress to a significant stenosis, setting the stage for an acute coronary syndrome.2 Unfortunately, no technique is available that can determine plaque composition in vivo. Many techniques to identify one or several determinants of plaque vulnerability are therefore the subject of intense investigation.3 Such techniques might not only be valuable for diagnosis of coronary artery disease but also for understanding the mechanisms by which the disease progresses or regresses.
Raman spectroscopy is a non-destructive optical technique that provides information about the contents of cholesterol and calcification in an atherosclerotic plaque.4 Recently, it has been shown that newly developed miniaturised fibreoptic probes are able to illuminate the blood vessel wall and to collect Raman scattered light from a blood filled environment,5 thereby providing an important step for the intravascular application of Raman spectroscopy.
Before clinical studies that apply Raman spectroscopy for the assessment of atherosclerosis can be initiated, several important issues need to be addressed. First, comparison with histology would provide data on the sensitivity and specificity of the clinical Raman system for detecting cholesterol and calcification; such data have not yet been obtained with the recently developed system which differs vastly from the Raman systems that were used in the initial studies.4,6,7 Second, from a clinical point of view, it is important to know the shortest collection time for acquisition of reliable Raman spectra.
In anticipation of future clinical trials investigating the potential of Raman spectroscopy for identifying plaque components in human coronary arteries, we addressed both these issues in the present study, using a newly developed fibreoptic based clinical system,5,8 which is capable of acquiring, correcting, analysing, and modelling Raman spectra from arterial tissue on-line and in real time.
METHODS
Tissue handling
Nine human coronary artery samples and four human aorta specimens were obtained at necropsy or from explanted hearts from patients undergoing heart transplantation. All samples were snap frozen in liquid nitrogen and stored at −80°C until use. For spectroscopic investigation, the arteries were thawed, opened longitudinally, flattened, and positioned on a cardboard surface covered by aluminium foil and attached to a cross table. The samples were immobilised using 0.5 French needles. The tip of the fibreoptic catheter was positioned perpendicular to the arterial surface. Subsequently, the catheter tip was lowered until it was in contact with the tissue. Spectra were obtained in 500 μm steps over the entire diameter of the sample (coronary artery samples) or the full width of the aortic specimen. For correlation with pathology, the initial position of the catheter was marked with a suture through the arterial wall.6 In addition, a schematic drawing of the arterial inner surface was provided to the pathologist. In all, 228 spectra were obtained (mean (SD), 17 (4) spectra per section). After spectroscopic measurements, the arterial samples were refrozen at −80°C and sent for histological evaluation.
Histology
Cross sections of the specimens (7 μm thick) were stained for general morphology (haematoxylin–eosin), collagen (picro–sirius red), and calcification (von Kossa). The frozen cryosections of the human specimens were stained with oil red O for detection of total lipids in the intima of the artery. The lipid content was assessed by the empty spaces in the picro–sirius red stain and the clearly visible lipid droplets in the oil red O stains.
Each millimetre of artery was scored for the presence of cholesterol and calcium salts by a histologist who was blinded to the outcome of the study. Quantities of cholesterol and calcification were assessed by eye and expressed as 0%, 5%, 10%, 20%, 30%, 40%, 50%, and > 60% of the arterial surface. In all, 114 sites were evaluated.
Data acquisition and spectral analysis
The compact clinical Raman system and fibreoptic catheters (Enviva Gaser 5, Visonex Inc, Atlanta, Georgia, USA) that were used in the experiments have been described in detail.8 In brief, the system consists of an 830 nm diode laser (Process Instruments diode laser, Salt Lake City, Utah, USA) which delivers its light through the 400 μm central core of an optical fibre to the arterial sample. Scattered Raman light is collected in seven surrounding collection fibres (300 μm). The collection fibres of the catheter were coupled to a modified dispersive imaging spectrometer (System 100, Renishaw, Wotton under Edge, Avon, UK), equipped with a thermoelectrically cooled deep depletion CCD camera.8
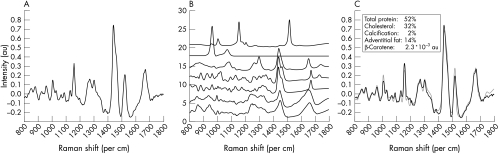
The software for real time data analysis has been described in detail.8 All spectra were analysed by means of a previously developed and validated least squares fitting routine,4 which uses Raman spectra of the main components of the arterial wall—that is, proteins, triglycerides, cholesterol, cholesteryl esters, calcium salts, and β carotene. To determine the molecular composition of the arterial wall, the relative weight percentages of the various components calculated by this fitting routine were then rescaled to add up to 100%.5 An example of the fitting procedure is shown in fig 1.
Figure 1.
Example of the Raman modelling procedure for quantification of proteins, calcification, and lipids, as described by Brennan and colleagues.4 (A) Raman spectrum after correction for background signal. This spectrum was obtained in 60 seconds from an atherosclerotic specimen using the fibreoptic catheter system. (B) Raman model spectra. From top to bottom: β carotene, calcification, adventitial fat, esterified cholesterol, cholesterol, delipidised atherosclerotic artery (protein 2), delipidised normal artery (protein 1). (C) Raman spectrum (black) and model fit (grey). The fit parameters are shown in the inset.
Correlation with histology
Contributions of two adjacent Raman spectra (0.5 mm) were combined to obtain an average value of total cholesterol (TC) or calcium salts (CS) per mm of coronary artery. These data (n = 114) were compared with the data provided by histology. Receiver operating characteristic (ROC) analysis was used to determine sensitivity and specificity of Raman spectroscopy for detecting cholesterol and calcification.
Mainly because of cutting artefacts in the calcified sections, it was difficult to define the exact place of the catheter; hence correlation could be improved by histological analysis of larger segments. To this purpose, contributions of four adjacent Raman spectra were averaged. This averaged contribution was subsequently compared with the histopathological diagnosis of 2 mm artery segments (n = 57).
Data acquisition measurements
From a total of 54 spots with varying histopathological characteristics, a set of Raman spectra was obtained with collection times varying between 1 and 60 seconds. At each collection time (1 s, 2 s, 3 s, . . .60 s), the Raman spectrum was fitted using the model developed by Brennan and colleagues.4 Fit contributions of protein, cholesterol, triglycerides, and calcification are presented as weight percentages. Fit contributions of β carotene are presented as arbitrary units.4
The fit contributions at shorter collection times were then compared with the data obtained from Raman spectra that were acquired in 60 seconds. For each component, correlation coefficients were determined. In addition, Bland–Altman analysis was used to determine whether systematic errors were encountered and whether the correlation between the shorter collection times and the reference data obtained in 60 seconds was appropriate.9
Statistical analysis
Statistical analysis was done with SPSS software (SPPS Inc, Chicago, Illinois, USA).
RESULTS
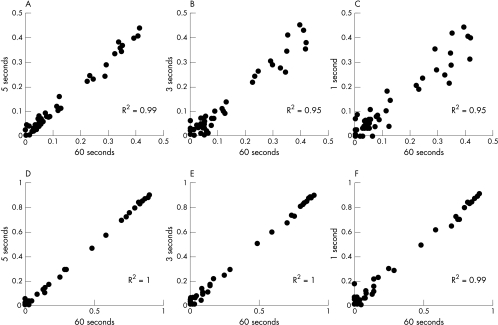
Figure 2 shows the correlation between the fit contributions of Raman spectroscopy for cholesterol (panels A to C) and calcification (panels D to F) in, respectively, 5 seconds, 3 seconds, and 1 second, and the fit contributions calculated from spectra that were obtained in 60 seconds. For quantification of calcification, the correlation coefficients for each of the acquisition times were excellent (R2 ⩾ 0.99), even for Raman data acquired with collection times as short as 1 second. For quantification of cholesterol, excellent correlation coefficients were achieved for data obtained with a minimum of 5 seconds of acquisition. Bland–Altman analysis showed that the differences between the values of cholesterol or calcification at short collection times (1–5 seconds) and at 60 seconds, did not vary in any systematic way.
Figure 2.
Correlation between the fit parameters calculated from Raman spectra acquired in 60 seconds (x axis), and the fit parameters calculated from Raman spectra that were acquired in respectively 5 seconds, 3 seconds, and 1 second (y axis). Panels A, B, and C: cholesterol; panels D, E, and F: calcification.
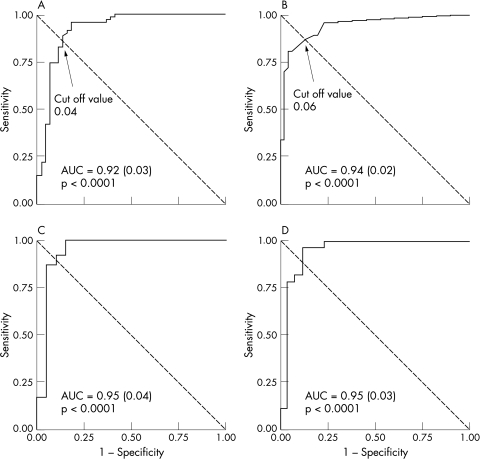
Areas containing large quantities of cholesterol (or calcification) on histological evaluation showed large contributions in the Raman spectrum. There was a highly significant correlation between the quantitative data from histological analysis and the data obtained by Raman spectroscopy (r = 0.68 and 0.71 for cholesterol and calcification, respectively; p < 0.0001). Qualitative detection of cholesterol and calcification was excellent: sensitivity and specificity for detecting even small pockets of cholesterol and calcification were high, as analysed by ROC analysis (fig 3, panels A and B). For cholesterol and calcification, areas under the ROC curve were ⩾ 0.92. ROC analysis was repeated on histological evaluations of 2 mm sections and resulted in areas under the curve of 0.95 (fig 3, panels C and D). For both evaluations, optimal cut off values for obtaining equal sensitivity and specificity were 4% for detecting cholesterol and 6% for detecting calcification. At these cut off values, sensitivity, specificity, and positive and negative predictive values are indicated in table 1.
Figure 3.
Receiver operating characteristic (ROC) curves for the detection of cholesterol (left hand panels) and calcification (right hand panels) by Raman spectroscopy. Panels A and B represent the accuracy if Raman diagnosis and histology had been made using 1 mm intervals. This evaluation was repeated for data averaged over 2 mm intervals (panels C and D). AUC: area under the curve.
Table 1.
Accuracy (%) for detection of cholesterol and calcification in 1 mm and 2 mm artery sections by Raman spectroscopy
| 1 mm section | 2 mm section | |||||
| Cholesterol | Calcification | β Carotene | Cholesterol | Calcification | β Carotene | |
| Sensitivity | 87 | 81 | 78 | 87 | 78 | 92 |
| Specificity | 86 | 95 | 95 | 95 | 97 | 95 |
| NPV | 91 | 94 | 97 | 97 | 94 | 95 |
| PPV | 80 | 86 | 74 | 80 | 86 | 92 |
Cut-off values of 4% (wt/wt), 6% (wt/wt) and 4.0×10−3 au were used for cholesterol, calcification, and β carotene, respectively. The contribution of β carotene was used as an indirect marker for detection of cholesterol (in bold).
NPV, negative predictive value; PPV, positive predictive value.
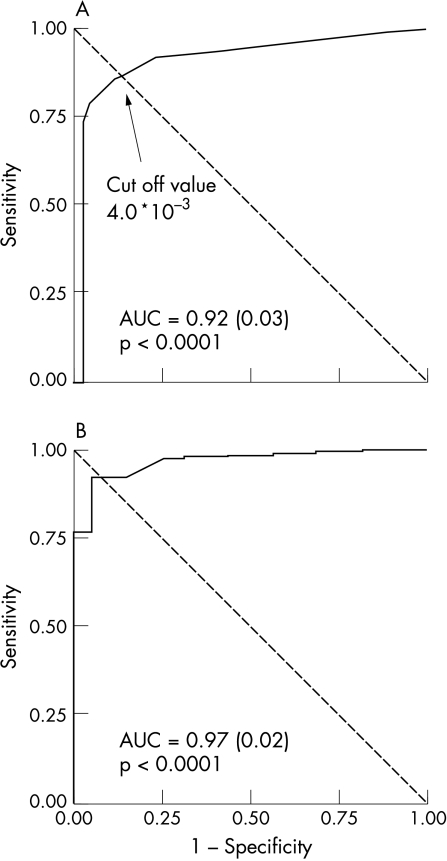
Figure 4 shows the results of ROC analysis when using β carotene (a vitamin with high lipid solubility) to predict the presence of cholesterol in the atherosclerotic lesion. Areas under the curve were 0.92 (1 mm intervals) and 0.97 (2 mm intervals). Sensitivity and specificity were comparable to those for cholesterol, and are indicated in the grey areas in table 1.
Figure 4.
Receiver operating characteristic (ROC) curves for the detection of cholesterol by evaluating the quantity of β carotene in the Raman spectra. The analysis is performed using 1 mm intervals (A) and 2 mm intervals (B) for Raman diagnosis and histology. AUC, area under the curve.
DISCUSSION
Our paper shows that Raman spectroscopy is capable of accurate detection of deposits of cholesterol and calcium salts in coronary arteries and aortic tissue, using a fibreoptic catheter and spectroscopic system that can be employed in a clinical setting. Acquisition times for reliable detection of cholesterol accumulation can be reduced to approximately 3–5 seconds, while accurate detection of calcification can be achieved in 1 second. There were highly significant correlations between the amount of cholesterol and calcification in the Raman spectrum and the quantity detected by histology. The sensitivity and specificity of Raman spectroscopy for detecting cholesterol and calcification within an atherosclerotic lesion were high and comparable with other spectroscopic techniques.10–12
At a cut off value of, respectively, 0.04 (wt/wt) and 0.06 (wt/wt), sensitivity and specificity for detecting cholesterol and calcification were approximately equal. This is comparable with results of Römer and colleagues,6 who found similar cut off values using large sensitive laboratory equipment and longer collection times. In this respect, it is remarkable that with a compact and relatively straightforward Raman system, equipped with catheters that are far from optimal, similar values were obtained. Apparently, the model developed by Brennan and colleagues4 is very robust, which shows promise for in vivo applications of the technique.
Positive and negative predictive values for detection of cholesterol obtained in the present study are comparable with corresponding values obtained with fibreoptic techniques such as near-infrared reflectance spectroscopy or time resolved fluorescence spectroscopy. However, the latter data were acquired with large laboratory equipment and have not yet been applied to an in vivo situation.11,13
Quantitative data provided by histology and Raman spectroscopy showed a highly significant but only moderate correlation. Previous Raman spectroscopic studies have achieved better correlations between Raman data and values obtained with chemical assays (r > 0.95) or oil red O staining (r = 0.87).4,14 Thus in our study the quantitative data of the Raman results do not always match the quantitative data obtained by histological evaluation. There may be several explanations for this discrepancy. First, when atherosclerotic tissue is stained, areas containing cholesterol stain entirely red with oil red O, and hence the area is quantified accordingly. However, only a small fraction of the atheroma contains cholesterol; other molecules, such as proteins and necrotic cell material, contribute to the Raman signal but are disregarded by the histology. Second, an important drawback of the use of the current prototype catheters is the fact that the excitation and collection volumes of the probe are poorly defined. Experiments in our laboratory have shown that the excitation/collection depth of the prototype catheters should be approximately 0.8 mm. A rough estimate of the sample volume of the catheter is approximately 0.8 × π × 0.52 = 0.63 mm3. However, owing to the organisation of the quartz fibres and the presence of complex filters in the tip of the catheters, the light reflected from the first 200 μm of tissue is not collected. Improvements to the catheters is a major facet of Raman research in our laboratory, and new catheters are under construction.
Our study shows that β carotene can be an accurate marker for the detection of plaque lipid. In previous studies, a close correlation has been found between the amount of lipid in a plaque and the peak intensity of (highly lipophylic) β carotene in the arterial Raman spectrum.7 The Raman scattering cross section of β carotene is much larger than that of cholesterol. Thus it will be easier to detect β carotene in a complex multicomponent Raman spectrum such as the spectrum of atherosclerotic artery tissue. Sensitivity and specificity for detecting lipid using β carotene were approximately 92% and 95%, respectively (2 mm artery segments). It would be of interest to investigate whether oral administration of carotenoids could facilitate the detection and quantification of cholesterol in an atherosclerotic plaque by Raman spectroscopy in vivo.
Conclusions
We have found that the sensitivity and specificity of catheter based Raman spectroscopy for on-line detection of cholesterol and calcification within a human atherosclerotic plaque are high, and that reliable Raman spectra can be obtained in approximately 3–5 seconds. Previous studies using Raman spectroscopy have shown that this catheter technique can be applied in vivo in the presence of blood flow.5,15 As soon as improved flexible (sideways viewing) Raman catheters are developed, Raman spectroscopy may be applied to assess plaque vulnerability and to evaluate the effects of drugs on plaque composition in vivo.
REFERENCES
- 1.Kullo IJ, Edwards WD, Schwartz RS. Vulnerable plaque: pathobiology and clinical implications. Ann Intern Med 1998;129:1050–60. [DOI] [PubMed] [Google Scholar]
- 2.Felton CV, Crook D, Davies MJ, et al. Relation of plaque lipid composition and morphology to the stability of human aortic plaques. Arterioscler Thromb Vasc Biol 1997;17:1337–45. [DOI] [PubMed] [Google Scholar]
- 3.Pasterkamp G, Falk E, Woutman H, et al. Techniques characterizing the coronary atherosclerotic plaque: influence on clinical decision making? J Am Coll Cardiol 2000;36:13–21. [DOI] [PubMed] [Google Scholar]
- 4.Brennan JF, Römer TJ, Lees RS, et al. Determination of human coronary artery composition by Raman spectroscopy. Circulation 1997;96:99–105. [DOI] [PubMed] [Google Scholar]
- 5.Buschman HP, Marple ET, Wach ML, et al. In vivo determination of the molecular composition of artery wall by intravascular Raman spectroscopy. Anal Chem 2000;72:3771–5. [DOI] [PubMed] [Google Scholar]
- 6.Römer TJ, Brennan JF, Puppels GJ, et al. Intravascular ultrasound combined with Raman spectroscopy to localize and quantify cholesterol and calcium salts in atherosclerotic coronary arteries. Arterioscler Thromb Vasc Biol 2000;20:478–83. [DOI] [PubMed] [Google Scholar]
- 7.Römer TJ, Brennan JF, Fitzmaurice M, et al. Histopathology of human coronary atherosclerosis by quantifying its chemical composition with Raman spectroscopy. Circulation 1998;97:878–85. [DOI] [PubMed] [Google Scholar]
- 8.Bakker Schut TC, Wolthuis R, Caspers PJ, et al. Real time tissue characterization on the basis of in vivo Raman spectra. J Raman Spectrosc 2002;33:580–5. [Google Scholar]
- 9.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;i:307–10. [PubMed] [Google Scholar]
- 10.Moreno PR, Lodder RA, Purushothaman KR, et al. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation 2002;105:923–7. [DOI] [PubMed] [Google Scholar]
- 11.Marcu L, Fishbein MC, Maarek JM, et al. Discrimination of human coronary artery atherosclerotic lipid-rich lesions by time-resolved laser-induced fluorescence spectroscopy. Arterioscler Thromb Vasc Biol 2001;21:1244–50. [DOI] [PubMed] [Google Scholar]
- 12.Richards-Kortum R, Mehta A, Hayes G, et al. Spectral diagnosis of atherosclerosis using an optical fiber catheter. Am Heart J 1989;118:381–91. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Geng YJ, Guo B, et al. Near-infrared spectroscopic characterization of human advanced atherosclerotic plaques. J Am Coll Cardiol 2002;39:1305–13. [DOI] [PubMed] [Google Scholar]
- 14.van de Poll SWE, Römer TJ, Volger OL, et al. Raman spectroscopic evaluation of the effects of diet and lipid-lowering therapy on atherosclerotic plaque development in mice. Arterioscler Thromb Vasc Biol 2001;21:1630–5. [DOI] [PubMed] [Google Scholar]
- 15.Van de Poll SWE, Buschman HPJ, Visser MJ, et al. Raman spectroscopy provides characterization of human atherosclerotic plaque composition in vivo [abstract]. J Am Col Cardiol 2000;35:52A. [Google Scholar]