Abstract
Many molecular and cellular mechanisms link inflammation and haemostatic mechanisms. Inflammation, and perhaps chronic infection, may play important roles in the initiation and progression of atherosclerosis. Atherosclerotic lesions are heavily infiltrated by cellular components associated with inflammation (macrophages and T lymphocytes), and acute plaque rupture is also associated with inflammatory components. Several markers of systemic inflammation may predict future cardiovascular events in apparently healthy subjects as well as in patients with chronic and acute syndromes. There may thus be therapeutic potential in modifying the atherosclerotic, vasomotor, and thrombotic components of ischaemic heart disease.
Keywords: inflammation, myocardial infarction, thrombosis
Coronary thrombosis is now widely recognised as a major cause of sudden cardiac death, acute myocardial infarction, and unstable angina pectoris. Inflammation is an important component of the atherosclerotic lesion. In this review we will discuss inflammatory mechanisms in relation to atherosclerosis and clinical coronary thrombosis.
The “response to injury” hypothesis postulates that endothelial dysfunction represents the initial step of atherogenesis and can be induced by haemodynamic forces, by a variety of vasoactive substances, by mediators from blood cells, and directly from risk factors for atherosclerosis.1 Upon activation, endothelial cells express various cellular adhesion molecules, cytokines, chemokines, and growth factors. Focal arterial inflammatory activity is one of the most prominent characteristics of the atherosclerotic process.2,3 Inflammation is also implicated in the pathogenesis of acute syndromes, as suggested by histological findings in unstable coronary plaques,4–6 evidence of systemic release of thromboxanes and leukotrienes,7–9 and the presence of activated circulating leucocytes.10,11 A process involving predominantly mononuclear leucocytes followed by fibrosis and finally tissue degeneration is common to many inflammatory disorders apart from atherosclerosis.
High sensitivity testing for C reactive protein (CRP)—a non-specific plasma marker of low grade systemic inflammation—has received much attention, and the results of several studies show a strong link between baseline elevations of CRP and the risk of future cardiac events.12–15 Other acute phase proteins also have prognostic significance in coronary artery disease.16,17 The leucocyte count and various inflammatory proteins such as fibrinogen,18,19 plasminogen activator inhibitor (PAI-1),16 von Willebrand factor,20 albumin,18 and various cytokines and adhesion molecules have been found to be independently associated with cardiovascular end points.21,22 Increased concentrations of interleukin 6 (IL-6), the major cytokine responsible for the acute phase response, are common in unstable patients and also correlate closely with prognosis.23 It has been suggested that the association between Chlamydia pneumoniae and clinical events24–27 may be because chlamydiae invade macrophages and exacerbate the inflammatory process within the plaque.28
CELLULAR AND MOLECULAR MECHANISMS INVOLVED IN INFLAMMATION
B and T lymphocyte in atherosclerosis
T cell adhesion to dysfunctional endothelium has been demonstrated in vivo and in vitro.29,30 T cells of both the helper (CD4+) and cytotoxic/suppressor types have been detected in human atheroma and have been shown to be immunologically activated.31 The first direct evidence for this activation was the demonstration of class II histocompatibility antigen expression on the surface of smooth muscle cells adjacent to the T lymphocytes in the lesions. This human lymphocyte antigen (HLA) expression is induced by interferon-γ, a product of activated T cells and natural killer cells. As natural killer cells are not found in complicated plaques, the only remaining source of interferon-γ is the adjacent activated T cells. The presence of activated T lymphocytes in the atherosclerotic plaque suggests a local immune response, and it has been postulated that such a response may be directed against local antigens in the plaque.32,33 Activated T lymphocytes secrete growth factors and cytokines that may affect other cell types and the process of atherosclerosis.
Monocytes and macrophages in atherosclerosis
Arterial leucocyte recruitment in early atherosclerosis also involves monocytes.34 Following endothelial adhesion and transmigration into the arterial intima, these cells express markers of activation such as HDL-DR, IL-2 receptor, and very late activation (VLA) antigen. Interleukins, complement factor fragments, and tumour necrosis factors (TNF) can enhance monocyte adhesiveness and chemotaxis and so form an amplification mechanism for recruitment of further monocytes into the lesion.35 Furthermore, released mitogens, such as macrophage derived growth factor, may play a key role in smooth muscle cell migration and subsequent proliferation.36
Activation of circulating leucocytes may be facilitated at the endothelium covering an atherosclerotic plaque, with upregulation of adhesion molecules and tethering of circulating cells. These inflammatory responses, possibly mediated by sCD40L, may further promote the infiltration of activated leucocytes into the atherosclerotic lesion, which in turn may directly activate smooth muscle cells, macrophages, and T cells inside the vessel wall.37,38 Lesional macrophages produce proteolytic enzymes which include members of the metalloproteinase family.3 Experimental studies39–41 have described constitutive expression of metalloproteinases by the macrophage foam cells within atheroma of hypercholesterolaemic rabbits. This macrophage related proteolysis within the atheroma contributes to weakness of the protective fibrous cap of the plaque and hence promotes the propensity of those plaques to rupture and trigger thrombosis. The interaction of macrophages with lymphocytes using CD40 and its ligand also upregulates metalloproteinases.37
Cytokines
Cytokines such as tumour necrosis factor α (TNFα) or IL-1 isoforms can stimulate the expression of IL-6, IL-8, and leucocyte adhesion molecules such as intercellular adhesion molecule (ICAM-1). These cytokines are produced by neutrophils and macrophages which are located in atheromatous plaques. They may be derived from non-vascular sources and reflect generalised inflammatory states, such as chronic infection, which have been linked to atherogenesis and its clinical manifestations.24 The contribution of vascular and extravascular sources of inflammatory cytokines may vary between individuals.
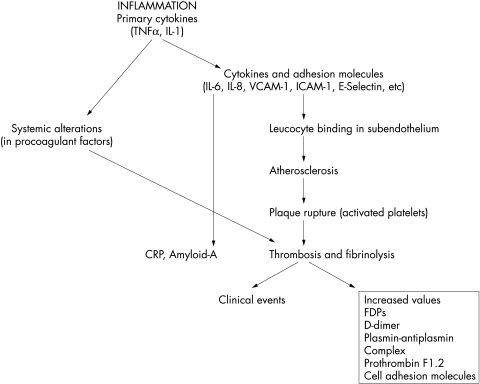
Primary cytokines (TNFα, IL-1) stimulate the production by endothelial and other cells of adhesion molecules, procoagulants, and other mediators that may be released in soluble form into circulating blood (fig 1). Primary cytokines also stimulate the production of messenger cytokine, IL-6, which induces expression of hepatic genes42 encoding acute phase reactants found in the blood, including CRP and serum amyloid A. In animal and human models of atherosclerosis, the first sign of disease activity is an upregulation of adhesion molecules such as the vascular cell adhesion molecule (VCAM-1), E-selectin, and ICAM.43 These molecules are instrumental in endothelial leucocyte binding and recruitment of leucocytes into the subendothelial tissue which are essential steps in the initial development of atherosclerotic lesion (fig 1). TNFα also stimulates the expression of IL-6.
Figure 1.
Links between inflammation and thrombotic mechanisms in atherosclerosis. Inflammation acting both locally and systemically may initiate atherosclerosis and promote thrombosis by weakening the fibrous cap of the atheromatous plaque leading to plaque rupture.
In patients with acute coronary syndromes, leucocyte–platelet adhesion is increased in circulating blood, suggesting an enhanced inflammatory response in patients with preinfarction angina.44 Binding to activated platelets induces IL-1β, IL-8, and monocyte chemoattractant protein 1 (MCP-1) in the leucocytes. These findings suggest that leucocyte–platelet adhesion may contribute to the regulation of inflammatory response in acute syndromes.
LIPOPROTEINS AND INFLAMMATION
Raised concentrations of low density lipoprotein (LDL) and possibly lipoprotein(a) (Lp(a)) may attract monocytes to adhere to endothelium and induce their transformation into macrophages.45 The proinflammatory effects of oxidised LDL involve peroxides and other reactive oxygen intermediates generated by the oxidation of LDL. These molecules activate nuclear transcription factor κB (NF-κB),45 which plays a key role in the orchestration of inflammatory and immune responses by controlling the transcription of the genes encoding several of the adhesion molecules, interleukins, TNFα, class II antigen, and antibodies. NF-κB recognises various activators, among which are the proinflammatory cytokines and CRP. Lp(a) is more abundant in atherosclerotic lesions from acute coronary syndromes and co-localises with macrophages in atherectomy specimens, suggesting interaction with inflammatory cells and extracellular matrices.46,47 Lp(a) enhances ICAM-1 expression partly by decreasing active transforming growth factor β.35
INFLAMMATION AND THROMBOSIS
Inflammatory cytokines modulate the homeostatic properties of the endothelium.1 The local effects of inflammatory cells on digestion of the fibrous cap lead to plaque disruption and thrombus formation. If the thrombi are small they may organise and contribute to the growth of the atherosclerotic plaque. If the thrombi are large or occlusive, they lead to the acute coronary syndromes.
Certain thrombogenic risk factors may modulate the degree of thrombogenicity and thereby determine growth of the plaque and the occurrence of the various acute coronary syndromes.16,48 Tissue factor is normally expressed in exposed intima and activates factor VII which in turn activates factors IX and X. Collagen in exposed intima binds von Willebrand factor, which mediates platelet adherence by binding to the glycoprotein Ib/V/IX platelet surface receptor complex under high shear stress conditions. von Willebrand factor itself is the carrier protein for factor VIII, an essential component of the amplifying mechanism of the factor X–Xa conversion. Furthermore, platelets activated by adhesion adhere to the other platelets through the glycoprotein IIb/IIIa receptor and its ligand, von Willebrand factor and fibrinogen. Such activated platelets release PAI-1, which locally inhibits the fibrinolytic mechanism.49
Inflammation may promote thrombosis by acting both locally and systemically. Local mechanisms include the cytokine stimulated expression of tissue factor by endothelial cells and macrophages. Indirectly, inflammation may act locally to induce thrombosis by weakening the fibrous cap of the atheromatous plaque, leading to plaque rupture. However, this role of inflammation, and specifically the role of macrophages, remains controversial.50
Inflammation can affect systemic haemostatic activity by IL-6 mediated stimulation of hepatocytes to produce acute phase reactants. These include certain coagulation factors, such as increased levels of fibrinogen and PAI-1, which induce a prothrombotic state (table 1).
Table 1.
Effects of inflammation on thrombosis–haemostasis
| 1 | Inflammatory cytokines modulate the haemostatic properties of the endothelium |
| 2 | Local effects of inflammatory cells on digestion of the fibrous cap lead to plaque disruption and thrombus formation |
| 3 | Inflammation can affect systemic haemostatic activity by IL-6 mediated stimulation of hepatocytes to produce acute phase reactants (coagulation factors, PAI-1) |
| 4 | Enhanced CD40L-CD40 interaction promotes thrombotic activity by enhancing tissue factor expression in macrophages and through the direct regulation of endothelial procoagulant activity |
| 5 | Activated platelets may mediate the homing of leucocytes by interaction with the subendothelial matrix under shear stresses |
| 6 | Oxidised LDL induces tissue factor expression in macrophages and decreases the anticoagulant activity of endothelium by interfering with thrombomodulin expression and inactivating tissue factor pathway inhibitor |
| 7 | Acute phase reactants are associated with an increased risk of future cardiovascular events which are mediated by acute thrombosis (for example, C reactive protein, fibrinogen, factor VIII) |
IL, interleukin; LDL, low density lipoprotein; PAI, plasminogen activator inhibitor.
An enhanced CD40L–CD40 interaction also promotes thrombotic activity by enhancing tissue factor expression in macrophages and through the direct regulation of endothelium procoagulant activity.38,51 Intravascular fibrinolysis induced by tissue type plasminogen activator may contribute to atherosclerosis by inducing P-selectin and platelet activating factor, as well as to plaque rupture by activating metalloproteinases52 (table 1).
Oxidised LDL also induces tissue factor expression in macrophages and decreases the anticoagulant activity of endothelium by interfering with thrombomodulin expression and inactivating tissue factor pathway inhibitor.53 Its expression is upregulated in circulating and endothelium adherent monocytes, and tissue factor has been found to be increased in coronary tissue of the culprit lesion from patients with unstable angina54–56 (table 1).
Recently, several studies have indicated that raised concentrations of CRP are associated with increased risks of future cardiovascular events. Initially, Liuzzo and colleagues and Haverkate and associates established the prognostic value of CRP in the setting of stable and unstable angina.14,57 Other acute phase reactants have also been used to indicate increased risk of cardiovascular events.58 Both fibrinogen59,60 and increased factor VII and VIII concentrations61,62 have been found to predict cardiovascular events in an independent manner. PAI-1 predicts second myocardial infarction in survivors of a first infarct.48
It is also now accepted that platelets may promote inflammatory responses. Studies have shown that activated platelets may mediate the homing of leucocytes by interaction with the subendothelial matrix under shear stresses that do not allow neutrophil adhesion.61,62 They may also contribute to the oxidative modification of LDL, provide a source of lipids for foam cell generation, and contribute to smooth muscle cell proliferation. Platelets from patients with unstable angina are characterised by notably decreased intracellular sCD40L concentrations as well as by decreased release of sCD40L (table 2).63
Table 2.
Platelets and inflammatory responses
| 1 | Activated platelets may mediate the homing of leucocytes by interaction with the subendothelial matrix under shear stresses that do not allow neutrophil adhesion |
| 2 | Activated platelets contribute to the oxidative modification of LDL |
| 3 | Activated platelets contribute to smooth muscle cell proliferation |
| 4 | Activated platelets contribute to inflammatory reactions by expressing and releasing CD40L, resulting in MMP activation and procoagulant activity |
LDL, low density lipoprotein; MMP, matrix metalloproteinase.
CHRONIC INFECTIONS AND INFLAMMATION
Experimental and clinical studies have suggested that there is a significant association between ischaemic heart disease and various infective diseases, both bacterial and viral, including cytomegalovirus, chronic bronchitis, and dental infections.64–66 More virulent Helicobacter pylori strains bearing the cytotoxin association gene A have a well recognised pathogenic role in peptic ulcer disease and gastric cancer67 and directly induce enhanced inflammation.68 A recent study69 supported the hypothesis that H pylori may influence atherogenesis through low grade, persistent inflammatory stimulation. It has been also shown that Chlamydia pneumoniae, cytomegalovirus, and H pylori may be detected within human atherosclerotic tissues.70–73 It has been hypothesised that these organisms may activate vessel associated leucocytes or lead to a transformation of vascular smooth muscle or endothelial cells.24 Another study74 showed associations of antibodies to C pneumoniae, H pylori, and cytomegalovirus with immune reactions to heat shock protein 60 and carotid or femoral atherosclerosis. Small trials27,75 have demonstrated that macrolide antibiotics which are active agents against C pneumoniae might reduce the cardiovascular event rate. Large studies are going on to explore the possible relation between antibiotics and clinical coronary events.
CLINICAL RELEVANCE
It has been suggested that inflammation with subsequent thrombus formation provides a potential explanation for the substantial percentage of patients who suffer an acute coronary event without evidence of any traditional risk factors for atherosclerosis.16,17,59,60,76,77 Lipid lowering treatment with pravastatin significantly reduces the serum concentration of inflammatory markers even when the lipid concentration has stabilised.78,79 Lipid lowering by dietary manipulation reduces proteolytic activity of macrophages and increases collagen content of established atheroma in rabbits.41 Lipid lowering treatment may also increase plaque stability by several other mechanisms, including reducing the size of the lipid core and decreasing inflammation, restoring endothelial function, decreasing tissue factor expression, and reducing the thrombosis.39–41,80 Abnormal coronary vasomotion and forearm blood flow abnormalities have been found in hypercholesterolaemic patients, and normalisation of function has been achieved by statin treatment.81–83 Chronic infections may be related to acute coronary syndromes, although their exact role is still to be validated.75,84 High doses of aspirin also reduce the risk of subsequent cardiovascular disease in apparently healthy men, and reduce their CRP concentrations.85 In a recent study from our group, thermal heterogeneity within human atherosclerotic coronary arteries was detected in vivo by application of a special thermographic catheter86; this correlated with the plasma CRP concentration.
CONCLUSIONS
Increasing numbers of molecular and cellular mechanisms have been identified which link inflammation and haemostatic mechanisms. Inflammation, and perhaps chronic infection, may play important roles in the initiation of atherosclerosis and progression to its final stages. Atherosclerotic lesions are heavily infiltrated by cellular components associated with inflammation (macrophages and T lymphocytes), and acute plaque rupture is also associated with inflammatory components. At present there is little evidence for a role of infections in acute coronary syndromes and thrombosis. Several markers of systemic inflammation may predict future cardiovascular events in apparently healthy subjects, as well as in patients with chronic and acute syndromes. There may therefore be therapeutic potential in modifying the atherosclerotic, vasomotor, and thrombotic components of ischaemic heart disease—directly or indirectly—by using anti-inflammatory, antioxidant, antibiotic, and lipid lowering agents. Future research efforts, and particularly randomised clinical trials, need to examine ways of modifying the links between inflammation, atherosclerosis, and thrombosis.
Abbreviations
CRP, C reactive protein
ICAM, intercellular adhesion molecule
IL, interleukin
LDL, low density lipoprotein
Lp(a), lipoprotein(a)
MCP, monocyte chemoattractant protein
NF-κB, nuclear transcription factor κB
PAI, plasminogen activator inhibitor
TGF, transforming growth factor
TNF, tumour necrosis factor
VCAM, vascular cell adhesion molecule
VLA, very late activation antigen
REFERENCES
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–9. [DOI] [PubMed] [Google Scholar]
- 2.Berliner JA, Navab M, Fogelman AM, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation and genetics. Circulation 1995;91:2488–96. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Geng YJ, Aikawa M, et al. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 1996;7:330–5. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, Takebayashi S, Kohchi K. Increased subendothelial infiltration of the coronary arteries with monocytes/macrophages in patients with unstable angina: histological data on 14 autopsied patients. Atherosclerosis 1987;68:191–7. [DOI] [PubMed] [Google Scholar]
- 5.van der Wal AC, Becker AE, van der Loos CM, et al. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 1994;89:36–44. [DOI] [PubMed] [Google Scholar]
- 6.Kohchi K, Takebayashi S, Hiroki T, et al. Significance of adventitial inflammation of the coronary artery in patients with unstable angina: results at autopsy. Circulation 1985;71:709–16. [DOI] [PubMed] [Google Scholar]
- 7.Stamenkovic I, Stegagno M, Wright KA, et al. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci USA 1988;85:1179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmer ME, Ware CF, Strominger JL. Characterization of a novel differentiation antigen complex recognized by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T-cells. J Immunol 1983;131:334–40. [PubMed] [Google Scholar]
- 9.Burmester GR, Jahn B, Gramatski M, et al. Activated T-cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the non-blastoid small T-cells of inflammation and normal T-cells activated in vitro. J Immunol 1984;133:1230–4. [PubMed] [Google Scholar]
- 10.Neumann FJ, Marx N, Gawaz M, et al. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation 1997;95:2387–94. [DOI] [PubMed] [Google Scholar]
- 11.Aukrust P, Muller FM, Ueland T, et al. Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina. Possible reflection of T-lymphocyte and platelet involvement into pathogenesis of acute coronary syndromes. Circulation 1999;100:614–20. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Cushman M, Stampfer MJ, et al. Plasma concentration of C-reactive protein and risks of developing peripheral vascular disease. Circulation 1998;97:425–8. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation 1998;97:2007–11. [DOI] [PubMed] [Google Scholar]
- 14.Liuzzo G, Biasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med 1994;331:417–24. [DOI] [PubMed] [Google Scholar]
- 15.Morrow DA, Rifai N, Antman EM, et al. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T, in acute coronary syndromes. J Am Coll Cardiol 1998;31:1460–5. [DOI] [PubMed] [Google Scholar]
- 16.Ridker P, Vaughan D, Stampfer M, et al. Endogenous tissue type plasminogen activator and risk of myocardial infarction. Lancet 1993;341:1165–8. [DOI] [PubMed] [Google Scholar]
- 17.Ridker P, Hennekens CH, Roitman-Johnson B, et al. Plasma concentration of soluble intercellular adhesion molecule-1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998;351:88–92. [DOI] [PubMed] [Google Scholar]
- 18.Danesh J, Collins R, Appleby P, et al. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease. JAMA 1998;279:1477–82. [DOI] [PubMed] [Google Scholar]
- 19.Koenig W. Fibrinogen and coronary risk. In: Fuster V, ed. Novel risk factors and the prevention of coronary heart disease. (Current cardiology reports.) Curr Sci 1999;1:112–18. [DOI] [PubMed] [Google Scholar]
- 20.Judan Vague I, Pyke SDM, Alessi M, et al. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. Circulation 1996;94:2057–63. [DOI] [PubMed] [Google Scholar]
- 21.Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis 1999;147:213–25. [DOI] [PubMed] [Google Scholar]
- 22.Fassbender K, Bertsch T, Mielke O, et al. Adhesion molecules in cerebrovascular diseases. Evidence for an inflammatory endothelial activation in cerebral large- and small-vessel disease. Stroke 1999;30:1647–50. [DOI] [PubMed] [Google Scholar]
- 23.Biasucci L, Vitelli A, Liuzzo G, et al. Elevated levels of interleukin-6 in unstable angina. Circulation 1996;94:874–7. [DOI] [PubMed] [Google Scholar]
- 24.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis: an assessment of the evidence and need for future research. Circulation 1997;96:4095–103. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JL, Muhlestein JB, Carlquist J, et al. Randomized secondary prevention trial of azithromycin in patients with coronary artery disease and serological evidence for Chlamydia pneumoniae infection. The azithromycin in coronary artery disease: elimination of myocardial infection with chlamydia (ACADEMIC) study. Circulation 1999;99:1540–7. [DOI] [PubMed] [Google Scholar]
- 26.Patel P, Mendall MA, Carrington D, et al. Association of helicobacter pylori and chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. BMJ 1995;311:711–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Leatham EW, Carrington D, et al. Elevated chlamydia pneumoniae antibodies, cardiovascular events and azithromycin in male survivors of myocardial infarction. Circulation 1997;96:404–7. [DOI] [PubMed] [Google Scholar]
- 28.Kol A, Sukhova GK, Lichtman AH, et al. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 1998;98:300–7. [DOI] [PubMed] [Google Scholar]
- 29.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 1989;135:169–75. [PMC free article] [PubMed] [Google Scholar]
- 30.Berman JW, Caldron TM. The role of endothelial cell adhesion molecules in the development of atherosclerosis. Cardiovasc Pathol 1992;1:17–28. [DOI] [PubMed] [Google Scholar]
- 31.Stemme S, Rymo L, Hannson GK. Polyclonal origin of T-lymphocytes in human atherosclerotic plaques. Lab Invest 1991;65:654–60. [PubMed] [Google Scholar]
- 32.Hansson GK, Holm J, Jonasson L. Detection of T-lymphocytes in the human atherosclerotic plaque. Am J Pathol 1989;135:169–75. [PMC free article] [PubMed] [Google Scholar]
- 33.Oksenberg JR, Starvi GT, Jeong MC, et al. Analysis of the T-cell receptor repertoire in human atherosclerosis. Cardiovasc Res 1997;36:256–67. [DOI] [PubMed] [Google Scholar]
- 34.Doukas J, Pober JS. Lymphocyte-mediated activation of cultured endothelial cells. J Immunol 1990;145:1088–98. [PubMed] [Google Scholar]
- 35.Davies MJ, Woolf N, Rowles P, et al. Lipid and cellular constituents of unstable human aortic plaques. Basic Res Cardiol 1994;89:33–9. [DOI] [PubMed] [Google Scholar]
- 36.Fuster V, Badimon L, Badimon JJ, et al. The pathogenesis of coronary artery disease and the acute coronary syndrome. N Engl J Med 1992;326:242–50/310–18. [DOI] [PubMed] [Google Scholar]
- 37.Schonbeck U, Mach F, Sukhova GK, et al. Regulation of matrix metalloproteinases expression in human vascular smooth muscle cells by T-lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res 1997;81:448–54. [DOI] [PubMed] [Google Scholar]
- 38.Mach F, Schonbeck U, Bonnefoy JY, et al. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation 1997;96:396–9. [DOI] [PubMed] [Google Scholar]
- 39.Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res 1995;77:863–8. [DOI] [PubMed] [Google Scholar]
- 40.Galis ZS, Sukhova GK, Lark MW, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aikawa M, Rabkin E, Okada Y, et al. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content in rabbit atheroma: a potential mechanism of lesion stabilization. Circulation 1998;97:2433–44. [DOI] [PubMed] [Google Scholar]
- 42.Amberger A, Maczek C, Jurgens G, et al. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones 1997;2:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Cybulski MI, Gimbrone MA, et al. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb 1993;13:197–204. [DOI] [PubMed] [Google Scholar]
- 44.Liuzzo G, Baisucci LM, Gallimore JR, et al. Enhanced inflammatory response in patients with preinfarction unstable angina. J Am Coll Cardiol 1999;34:1696–703. [DOI] [PubMed] [Google Scholar]
- 45.Hajjar DP, Haberland ME. Lipoprotein trafficking in vascular cells: molecular Trojan horses and cellular saboteurs. J Biol Chem 1997;272:22975–8. [DOI] [PubMed] [Google Scholar]
- 46.Shindo J, Ishibashi T, Kijima M, et al. Increased lipoprotein (a) deposition and macrophage number of atherectomy in acute coronary syndromes. Circulation 1998;98(suppl):I-46. [Google Scholar]
- 47.Dangas G, Mehran R, Harpel PC, et al. Lipoprotein (a) and inflammation in coronary atheroma: association with the severity of clinical presentation. J Am Coll Cardiol 1998;32:2035–42. [DOI] [PubMed] [Google Scholar]
- 48.Hamsten A, deFaire U, Walldins G, et al. Plasminogen activator inhibitor in plasma as risk factor for recurrent myocardial infarction. Lancet 1987;2:3–9. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno O, Hojo Y, Ikeda U, et al. Assessment of coagulation and platelet activation in coronary sinus blood induced by transcatheter coronary intervention for narrowing of the left anterior descending coronary artery. Am J Cardiol 2000;85:154–60. [DOI] [PubMed] [Google Scholar]
- 50.Maseri A. Inflammation, atherosclerosis and ischemic events – exploring the hidden side of the moon. N Engl J Med 1997;336:1014–15. [DOI] [PubMed] [Google Scholar]
- 51.Miller DL, Yaron R, Yellin MJ. CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J Leukoc Biol 1988;63:373–9. [DOI] [PubMed] [Google Scholar]
- 52.Lacoste L, Lam JY, Hung J, et al. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation 1995;92:3172–7. [DOI] [PubMed] [Google Scholar]
- 53.Aikawa M, Voglic SJ, Sugiyama S, et al. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation 1999;100:1215–22. [DOI] [PubMed] [Google Scholar]
- 54.Leathman EW, Bath PM, Tooze JA, et al. Increased monocyte tissue factor expression in coronary artery disease. Br Heart J 1995;73:10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo SK, Cheung A, Zheng Q, et al. Induction of tissue factor on monocytes by adhesion to endothelial cells. J Immunol 1995;154:4768–77. [PubMed] [Google Scholar]
- 56.Annex BH, Denning SM, Keith MC, et al. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation 1995;91:619–22. [DOI] [PubMed] [Google Scholar]
- 57.Haverkate F, Thompson S, Duckert F. Haemostasis factors in angina pectoris: relation to gender, age, and acute phase reaction. Thromb Haemost 1995;73:561–7. [PubMed] [Google Scholar]
- 58.Tracy R. Atherosclerosis, thrombosis and inflammation: a question of linkage. Fibrinolysis Proteolysis 1997;II(suppl 1):137–42. [Google Scholar]
- 59.Ernst E, Resch K. Fibrinogen as a cardiovascular risk factor: a meta-analysis and review of the literature. Ann Intern Med 1993;118:956–63. [DOI] [PubMed] [Google Scholar]
- 60.Folsom A, Wu K, Rosamond W, et al. Prospective study of hemostatic factors and incidence of coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Circulation 1997;96:1102–8. [DOI] [PubMed] [Google Scholar]
- 61.Badimon L, Badimon JJ. Mechanisms of arterial thrombosis in non-parallel streamlines: platelets grow at the apex of stenotic severely injured vessel wall. Experimental study in the pig model. J Clin Invest 1989;84:1134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lassila R, Badimon JJ, Valibhajosula S, et al. Dynamic monitoring of platelets deposition on severely damaged vessel wall in flowing blood. Effects of different stenosis on thrombus growth. Arteriosclerosis 1990;10:306–15. [DOI] [PubMed] [Google Scholar]
- 63.Mach F, Schonbeck U, Libby P. CD40 signaling in vascular cells. A key role in atherosclerosis? Atherosclerosis 1998;137:S89–95. [DOI] [PubMed] [Google Scholar]
- 64.Benditt EP, Barrett T, McDougall JK. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci USA 1983;80:6386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jousilahti P, Vartiaainen E, Tuomilehto J, et al. Symptoms of chronic bronchitis and the risk of coronary disease. Lancet 1996;348:567–72. [DOI] [PubMed] [Google Scholar]
- 66.Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction. BMJ 1989;298:779–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection by Helicobacter pylori with strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 1995;55:2111–15. [PubMed] [Google Scholar]
- 68.Peek RM, Miller GG, Tham KT, et al. Heightened inflammatory response and cytokine-expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest 1995;73:760–70. [PubMed] [Google Scholar]
- 69.Pasceri V, Cammarota G, Patti G, et al. Association of virulent Helicobacter pylori strains with ischemic disease. Circulation 1998;97:1675–9. [DOI] [PubMed] [Google Scholar]
- 70.Grayston JT, Kuo CC, Coulson AS, et al. Chlamydia pneumoniae (TWAR) in the atherosclerosis of the carotid artery. Circulation 1995;92:3397–400. [DOI] [PubMed] [Google Scholar]
- 71.Muhlestein JB, Hammond EH, Carlquist JF, et al. Increased incidence of Chlamydia species within the coronary arteries of patients with symptomatic atherosclerotic versus other forms of cardiovascular disease. J Am Coll Cardiol 1996;27:1555–61. [DOI] [PubMed] [Google Scholar]
- 72.Melnick JL, Hu C, Burek J, et al. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J Med Virol 1994;42:170–4. [DOI] [PubMed] [Google Scholar]
- 73.Wu TC, Hruban RH, Ambinder RF, et al. Demonstration of cytomegalovirus nucleic acids in the coronary arteries of transplanted hearts. Am J Pathol 1992;140:739–47. [PMC free article] [PubMed] [Google Scholar]
- 74.Mayr M, Kiechl S, Willeit J, et al. Infections, immunity and atherosclerosis. Associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation 2000;102:833–9. [DOI] [PubMed] [Google Scholar]
- 75.Gurfinkel E, Bozovich G, Daroca A, et al, for the ROXIS Study Group. Randomized trial of roxithromycin in non-Q-wave coronary syndromes: ROXIS pilot study. Lancet 1997;350:404–7. [DOI] [PubMed] [Google Scholar]
- 76.Koenig W, Sund M, Frohlich M, et al. C-reactive protein a sensitive marker of inflammation predicts future risk of coronary heart disease in initially healthy middle-aged men. Circulation 1999;99:237–42. [DOI] [PubMed] [Google Scholar]
- 77.Ridker PM, Buring JE, Shih J, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998;98:731–3. [DOI] [PubMed] [Google Scholar]
- 78.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med 1966;335:1001–9. [DOI] [PubMed] [Google Scholar]
- 79.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol level. Circulation 1998;98:839–44. [DOI] [PubMed] [Google Scholar]
- 80.Davies MJ. Reactive oxygen species, metalloproteinases and plaque stability. Circulation 1998;97:2382–3. [DOI] [PubMed] [Google Scholar]
- 81.Egashira K, Inou T, Yamada A, et al. Impaired coronary blood flow response to acetylcholine in patients with risk factors and proximal atherosclerotic lesions. J Clin Invest 1993;91:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tousoulis D, Gorog D, Crake T, et al. Vasomotor responses of coronary stenoses to acetylcholine and their relation to serum lipid levels in stable angina pectoris. Am J Cardiol 1999;83:1606–10. [DOI] [PubMed] [Google Scholar]
- 83.Mougenot N, Lesnik P, Ramirez-Gil JF, et al. Effect of the oxidation state of LDL on the modulation of arterial response in vivo. Atherosclerosis 1997;133:183–92. [DOI] [PubMed] [Google Scholar]
- 84.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ 2000;321:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9. [DOI] [PubMed] [Google Scholar]
- 86.Stefanadis C, Diamantopoulos L, Vlachopoulos C, et al. Thermal heterogeneity within human atherosclerotic coronary arteries detected in vivo: a new method of detection by application of a special thermography catheter. Circulation 1999;99:1965–71. [DOI] [PubMed] [Google Scholar]