The aetiology based classification of pericardial disease comprises: infectious pericarditis; pericarditis in systemic autoimmune diseases; type 2 (auto)immune pericarditis; metabolic disorders; trauma; tumours; pericardial cysts; and congenital defects.1 This classification has major therapeutic consequences that will be elaborated upon in this article, with the focus on practical management of pericardial syndromes and specific underlying diseases.
DIAGNOSTIC ASPECTS
Pericardial syndromes
The diagnosis of acute pericarditis relies on clinical findings, ECG changes, and echocardiography (table 1).2,3 Chronic pericardial inflammation includes effusive, adhesive, and constrictive forms, lasting three months or more. Recurrent pericarditis may be intermittent (symptom-free interval without treatment) or incessant (discontinuation of anti-inflammatory treatment always ensures a relapse).
Table 1.
| Diagnostic measure | Characteristic findings |
| Obligatory | |
|
Pericardial rub (mono-, bi-, or triphasic) |
|
Stage I: anterior and inferior concave ST segment elevation. PR segment deviations opposite to P wave polarity |
| Early stage II: all ST junctions return to the baseline. PR segments deviated | |
| Late stage II: T waves progressively flatten and invert | |
| Stage III: generalised T wave inversions in most or all leads | |
| Stage IV: ECG returns to prepericarditis state | |
|
Effusion types B-D (Horowitz) |
| Signs of tamponade | |
|
ESR, CRP, LDH, leucocytes (inflammation markers) |
| Troponin I, CK-MB (markers of myocardial involvement) | |
|
Ranging from normal to “water bottle” shape |
| Performed primarily to reveal pulmonary or mediastinal pathology | |
| Mandatory in tamponade, optional in large/recurrent effusions or if previous tests inconclusive in small effusions | |
|
PCR and histochemistry for aetiopathogenetic classification of infection or neoplasia |
| Optional or if previous tests inconclusive | |
|
Effusions, peri-, and epicardium |
|
Effusions, peri-, and epicardium |
|
Establishing the specific aetiology |
*Typical lead involvement: I, II, aVL, aVF, and V3–V6. The ST segment is always depressed in aVR, frequently in V1, and occasionally in V2. Occasionally, stage IV does not occur and there are permanent T wave inversions and flattenings. If ECG is first recorded in stage III, pericarditis cannot be differentiated by ECG from diffuse myocardial injury, “biventricular strain” or myocarditis. ECG in early repolarisation is very similar to stage I. Unlike stage I, this ECG does not acutely evolve and J point elevations are usually accompanied by a slur, oscillation, or notch at the end of the QRS just before and including the J point (best seen with tall R and T waves—large in early repolarisation pattern). Pericarditis is likely if in lead V6 the J point is > 25% of the height of the T wave apex (using the PR segment as a baseline).
CK-MB, creatine kinase MB; CRP, C reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase;
MRI, magnetic resonance imaging; PCR, polymerase chain reaction.
Pericardial effusion occurs as transudate (hydropericardium), exudate, pyopericardium or haemopericardium, or a mixture of these. Large effusions generally indicate more serious disease and are common with neoplasia, tuberculosis, hypercholesterolaemia, uraemic pericarditis, myxoedema, and parasitoses.2,4 Patients can be asymptomatic if effusion develops slowly. Many pregnant women develop a minimal to moderate clinically silent hydropericardium by the third trimester. Fetal pericardial fluid can be detected by echocardiography after 20 weeks’ gestation and is normally 2 mm or less in depth. More fluid should raise questions of hydrops fetalis, Rh disease, hypoalbuminaemia, and immunopathy or maternally transmitted mycoplasmal or other infections, and neoplasia.3
Echocardiography reveals the size of effusions: (1) small (echo-free space in diastole < 10 mm); (2) moderate (at least ⩾ 10 mm posteriorly); (3) large (⩾ 20 mm); or (4) very large (⩾ 20 mm with compression of the heart).2 Presence of fibrin, clot, tumour, air, and calcium can also be detected. Pericardial effusion must be differentiated from pleural fluid, ascites, atelectasis, or epicardial fat. Transoesophageal echocardiography is useful in loculated pericardial effusions, intrapericardial clots, metastases, and pericardial thickening.
Cardiac tamponade is a medical emergency and its diagnosis is of major clinical importance (table 2).3 Pericardial disease of almost any aetiology can produce cardiac tamponade by effusion accumulation and increased intrapericardial pressure. “Surgical tamponade” (for example, haemorrhage) can quickly overwhelm compensatory mechanisms. Slowly developing “medical tamponade” may first appear with an effusion of 500–2000 ml.
Table 2.
Diagnosis of cardiac tamponade3
|
Raised systemic venous pressure*, hypotension†, pulsus paradoxus‡, tachycardia§, dyspnoea or tachypnoea with clear lungs |
|
Drugs (cyclosporin, anticoagulants, thrombolytics, etc), recent cardiac surgery, indwelling instrumentation, blunt chest trauma, malignancies, connective tissue disease, renal failure, septicaemia¶ |
|
Can be normal or non-specifically changed (ST-T wave), electrical alternans (QRS, rarely T), bradycardia (end stage), electromechanical dissociation (agonal phase) |
|
Enlarged cardiac silhouette with clear lungs |
|
Diastolic collapse of the (1) anterior RV free wall**, RA collapse, LA and rarely LV collapse, increased LV diastolic wall thickness “pseudohypertrophy”, VCI dilatation (no collapse in inspirium), “swinging heart” |
|
Tricuspid flow increases and mitral flow decreases during inspiration (reverse in expiration) |
| Systolic and diastolic flows are reduced in systemic veins in expirium and reverse flow with atrial contraction is increased | |
|
Large respiratory fluctuations in mitral/tricuspid flows |
|
|
|
Atrial collapse and small hyperactive ventricular chambers |
|
Coronary compression in diastole |
*Jugular venous distension is less notable in hypovolaemic patients or in “surgical tamponade”. An inspiratory increase or lack of fall of the pressure in the neck veins (Kussmaul sign), when verified with tamponade, or after pericardial drainage, indicates effusive–constrictive disease.
†Heart rate is usually >100 beats/min, but may be lower in hypothyroidism and in uraemic patients.
‡Pulsus paradoxus is defined as a drop in systolic blood pressure >10 mm Hg during inspiration whereas diastolic blood pressure remains unchanged. It is easily detected by simply feeling the pulse, which diminishes significantly during inspiration. Clinically significant pulsus paradoxus is apparent when the patient is breathing normally. When this sign is present only in deep inspiration it should be interpreted with caution. The magnitude of pulsus paradoxus is evaluated by sphygmomanometry. If the pulsus paradoxus is present, the first Korotkoff sound is not heard equally well throughout the respiratory cycle, but only during expiration at a given blood pressure. The blood pressure cuff is therefore inflated above the patient’s systolic pressure. Then it is slowly deflated while the clinician observes the phase of respiration. During deflation, the first Korotkoff sound is intermittent. Correlation with the patient’s respiratory cycle identifies a point at which the sound is audible during expiration, but disappears when the patient breathes in. As the cuff pressure drops further, another point is reached when the first blood pressure sound is audible throughout the respiratory cycle. The difference in systolic pressure between these two points is the clinical measure of pulsus paradoxus. Pulsus paradoxus is absent in tamponade complicating atrial septal defect and in patients with significant aortic regurgitation.
§Occasional patients are hypertensive, especially if they have pre-existing hypertension.
¶Febrile tamponade may be misdiagnosed as septic shock.
**Right ventricular collapse can be absent in raised right ventricular pressure and right ventricular hypertrophy or in right ventricular infarction.
††If after drainage of pericardial effusion intrapericardial pressure does not fall below atrial pressure, then effusive–constrictive disease should be considered.
LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; VCI, inferior vena cava.
Constrictive pericarditis causes impaired filling of the ventricles and reduced ventricular function (table 3).3,5 Tuberculosis, mediastinal irradiation, and previous cardiac surgery are frequent causes. Differential diagnosis must include pulmonary embolism, right ventricular infarction, pleural effusion, chronic obstructive lung disease, and restrictive cardiomyopathy. The best way to distinguish constrictive pericarditis from restrictive cardiomyopathy is by analysing respiratory and preload changes using Doppler/tissue Doppler echocardiography.3 Physical findings, ECG, chest radiography, computed tomography/magnetic resonance imaging (CT/MRI), haemodynamics, and endomyocardial biopsy may be helpful as well.
Table 3.
|
Severe chronic systemic venous congestion associated with low cardiac output, including jugular venous distension, hypotension with a low pulse pressure, abdominal distension, oedema, and muscle wasting |
|
Can be normal, or reveal low QRS voltage, generalised T wave inversion/flattening, LA abnormalities, atrial fibrillation, atrioventricular block, intraventricular conduction defects, or rarely pseudoinfarction pattern |
|
Pericardial calcifications, pleural effusions |
|
Pericardial thickening and calcifications* as well as the indirect signs of constriction:
|
|
Restricted filling of both ventricles with respiratory variation >25% over the AV valvesঠ|
|
Measurement of the pericardial thickness |
|
Measurements of the pericardial fibrosis and thickening |
| Cardiac catheterisation: | “Dip and plateau” or “square route” sign in the pressure curve of the right and/or left ventricle |
| Equalisation of pressures in the range of 5 mmHg or less§ | |
|
The reduction of RV and LV size and increase of RA and LA size |
| During diastole a rapid early filling with stop of further enlargement (“dip-plateau”) | |
|
In all patients over 35 years and in patients with a history of mediastinal irradiation, regardless of the age. |
*Thickening of the pericardium is not always equal to constrictive physiology.
†Diagnosis is difficult in atrial fibrillation. Hepatic diastolic vein flow reversal in expirium is observed even when the flow velocity pattern is inconclusive.
‡Patients with increased atrial pressures or mixed constriction and restriction demonstrate <25% respiratory changes. A provocation test with head-up tilting or sitting position with decrease of preload may unmask the constrictive pericarditis.
§In the early stage or in the occult form, these signs may not be present and the rapid infusion of 1–2 litres of normal saline may be necessary to establish the diagnosis. Constrictive haemodynamics may be masked or complicated by valvar and coronary artery disease.
¶In chronic obstructive lung disease mitral in-flow velocity will decrease nearly 100% during inspiration and increase during expiration. The mitral E velocity is highest at the end of expiration (in constrictive pericarditis mitral E velocity is highest immediately after start of expiration). In addition, superior vena cava flow increases with inspiration in chronic obstructive lung disease, whereas it does not change significantly with respiration in constrictive pericarditis.
LA, left atrium; LV, left ventricle; MRI, magnetic resonance imaging; RA, right atrium; RV, right ventricle, TOE, transoesophageal echocardiography.
How to establish the aetiology?
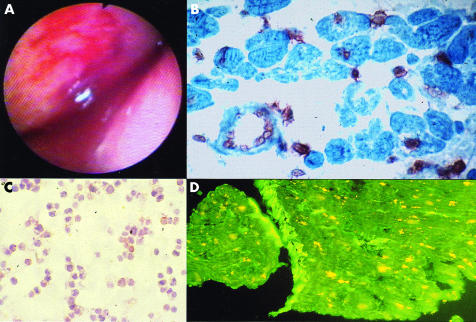
Viral pericarditis is the most common infectious disease of the pericardium and is caused by direct viral attack (enterovirus, adenovirus, cytomegalovirus (CMV), Ebstein-Barr virus, herpes simplex virus, influenza virus, parvo B19, hepatitis C virus, HIV, etc), the immune response, or both.6 Initial presentation is most frequently the syndrome of acute pericarditis, often resolving within two weeks, but with up to 50% recurring.3 The acute effusive form, particularly after tamponade, is associated with constriction more often than “dry” pericarditis. Arrhythmias or conduction defects indicate myocarditis or other concomitant heart disease. Diagnosis is not possible without the evaluation of pericardial effusion and/or pericardial/epicardial tissue by polymerase chain reaction (PCR) or in situ hybridisation (fig 1). A fourfold rise in serum antiviral antibodies is suggestive but not diagnostic per se.
Figure 1.
Viral pericarditis: (A) pericardioscopy findings; (B) epicardial in situ hybridisation positive for cytomegalovirus; (C) cytology of pericardial effusion; (D) epicardial immunofluorescence staining.
The diagnosis of autoreactive pericarditis is established by: (1) an increased number of lymphocytes/mononuclear cells > 5000/mm3 (autoreactive lymphocytic), or the presence of antibodies against heart muscle tissue in the pericardial fluid (autoreactive antibody mediated); (2) signs of myocarditis on epicardial/endomyocardial biopsies (⩾ 14 cells/mm2); (3) exclusion of active viral infection in pericardial effusion and endomyocardial/epimyocardial biopsies (no virus isolation, no immunoglobulin IgM titre against cardiotropic viruses in pericardial effusion, and negative PCR for major cardiotropic viruses); (4) tuberculosis, Borrelia burgdorferi, Chlamydia pneumoniae, and other bacterial infection excluded by PCR and/or cultures; (5) no neoplastic infiltration in pericardial effusion or tissue; and (6) exclusion of uraemia, systemic, and metabolic disorders.7
Despite treatment, purulent pericarditis is fatal in up to 40% of patients (tamponade, toxicity, constriction). Predisposing conditions are pre-existing pericardial effusion, immunosuppression, and chronic diseases (alcoholism, rheumatoid arthritis, etc). Pericardial fluid should undergo urgent Gram, acid-fast, and fungal staining, followed by cultures for aerobes and anaerobes of the pericardial and body fluids. Purulent effusions have significantly lower fluid glucose concentrations (mean (SD) 47.3 (25.3) v 102.5 (35.6) mg/dl) and fluid to serum ratios (0.28 (0.14) v 0.84 (0.23) mg/dl), than non-infectious effusions.8
In the last decade tuberculous pericarditis in developed countries has been primarily seen in immunocompromised patients (AIDS).9 Pericardial constriction occurs in 30–50% of cases and mortality in untreated disease approaches 85%. The absolute criteria for diagnosis are the identification of Mycobacterium tuberculosis in the pericardial fluid or tissue, and/or the presence of caseous granulomas in the pericardium.3 However, pericarditis in proven extracardiac tuberculosis is strongly suggestive of a tuberculous aetiology. A systematic, multiple diagnostic approach is essential (sputum cultures, ELISPOT skin test, analyses of pericardial effusion by acid-fast staining, mycobacterial culture or radiometric growth detection (for example, BACTEC-460), adenosine deaminase (ADA), pericardial lysozyme, and PCR for M tuberculosis).3,10 High ADA (> 40 U/l) in pericardial effusion is diagnostic for tuberculous pericarditis (93% sensitivity, 97% specificity) and prognostic for pericardial constriction.11 PCR is as sensitive (75% v 83%), but more specific (100% v 78%) for tuberculous pericarditis than ADA. Both pericardioscopy and pericardial biopsy have further improved the diagnostic accuracy for tuberculous pericarditis.12
Two forms of pericarditis have been described in renal failure:
Uraemic pericarditis—fibrinous inflammation with adhesions between the thickened pericardial membranes (“bread and butter” appearance) caused by the high degree of azotemia (blood urea nitrogen usually > 60 mg/dl) in advanced renal failure before dialysis is started or shortly thereafter.
Dialysis associated pericarditis—patients on maintenance haemodialysis, and occasionally with chronic peritoneal dialysis caused by inadequate dialysis and/or fluid overload.
After renal transplantation pericarditis may be caused by uraemia or infections (for example, CMV).
The post-cardiac injury syndrome develops within days to months after cardiac/pericardial injury. Unlike the post-myocardial infarction syndrome, it acutely provokes a greater antiheart antibody response (antisarcolemmal and antifibrillary). These antibodies appear to be pathogenic or may act in the presence of a dormant or concurrent viral infection.
Pericarditis may occur “early” (pericarditis epistenocardica) or be “delayed” (Dressler’s syndrome) after myocardial infarction. Epistenocardiac pericarditis, caused by direct exudation, occurs in the first several days of almost half of transmural myocardial infarctions, although early thrombolytic treatment has decreased its incidence. Dressler’s syndrome occurs from one week to several months after myocardial infarction (also in subendocardial forms) with manifestations similar to the post-cardiac injury syndrome. Cardiac tamponade may occur relatively early. Late constriction is rare but not surprising because of intrapericardial organisation of exudate and blood that can also produce loculated effusions. If the diagnosis of acute myocardial infarction is mistaken, thrombolysis may produce tamponade from inflamed pericardium or aortic dissection.
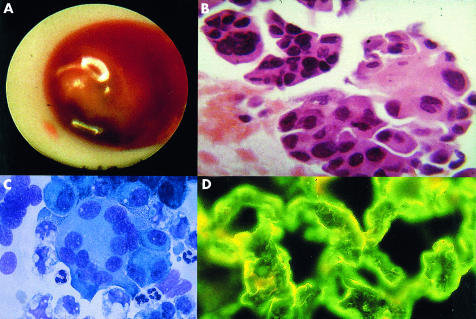
Neoplastic pericarditis is 40 times more often caused by secondary than primary malignancies, most frequently by lung cancer, breast cancer, malignant melanoma, lymphomas, and leukemias. Effusions may be small or large with an imminent tamponade or constriction. The diagnosis is based on pericardial fluid cytology and pericardial/epicardial biopsy findings (fig 2). CT/MRI can reveal localisation of the primary tumour or the metastases. Pericardial fluid cytology is positive in 75–87% and pericardial biopsy in 27–65% of patients with malignant pericardial disease.13 However, pericardial biopsies guided by pericardioscopy have a diagnostic value of 93.3–97%.12,14 Increased concentrations of specific tumour markers (for example, carcinoembryonic antigen (CEA), α-feto protein (AFP), carbohydrate antigens CA 125, CA 72-4, CA 15-3, CA 19-9, CD-30, CD-25) may also suggest the diagnosis. Of note, in 60% of the patients with documented malignancy, pericardial effusion is caused by non-malignant diseases—for example, radiation pericarditis or opportunistic infections.13
Figure 2.
Neoplastic pericarditis in Hodgkin’s disease: (A) pericardioscopy findings; (B) epicardial histology; (C) cytology of pericardial effusion; (D) epicardial immunofluorescence.
Fungal pericarditis occurs mainly in immunocompromised patients (Candida, Aspergillus, Blastomyces, Nocardia, and Actinomyces species) or in the course of endemic fungal infections (Histoplasma, coccidioides).2 The clinical picture comprises the full spectrum of pericardial diseases including fungal myocarditis. Diagnosis is obtained by staining and culturing pericardial fluid or tissue, but antifungal antibodies in serum are also helpful.3
Chylopericardium refers to a communication between the pericardial sac and the thoracic duct caused by congenital anomalies, trauma, mediastinal lymphangiomas, lymphangiomatous hamartomas, mediastinal or pericardial lymphangiectasis, obstruction or anomalies of the thoracic duct, or caused iatrogenically during surgery. Infection, tamponade or constriction may aggravate the prognosis. The chylous nature of the fluid is confirmed by its alkaline reaction, specific gravity between 1010 and 1021, Sudan III stain for fat, and the high concentrations of triglycerides (5–50 g/l) and protein (22–60 g/l). Enhanced CT, alone or combined with lymphography, is a reliable diagnostic tool for identifying not only the location of the thoracic duct but also its lymphatic connection to the pericardium.3
THERAPEUTIC ASPECTS
Whenever possible, treatment should be aimed at the underlying aetiology. Hospitalisation is warranted for most patients to determine the aetiology, observe for cardiac tamponade, and start anti-inflammatory and symptomatic treatment. Indications for specific therapeutic modalities, pericardiocentesis, intrapericardial treatment, and surgery are described in this section.
Chest pain and pericardial inflammation
Non-steroidal anti-inflammatory drugs (NSAIDs) are the mainstay of treatment. Ibuprofen (300–800 mg every 6–8 hours) is preferred because of its minimal side effects, favourable effect on the coronary flow, and the large dose range.3 Nevertheless, gastrointestinal protection should be provided. There is increasing evidence that colchicine (0.5 mg twice daily) added to an NSAID or as monotherapy is also effective for the initial attack and prevention of recurrences.15 Colchicine is well tolerated with fewer side effects than NSAIDs. Caution is necessary in pregnancy—high dose aspirin may prematurely close the ductus arteriosus and colchicine is contraindicated. Systemic corticosteroid treatment (1–1.5 mg/kg/day) should be restricted to connective tissue diseases, uraemic pericarditis, and severely symptomatic recurrent pericarditis not responding to colchicine. For those patients not responding adequately, azathioprine or cyclophosphamide can be added.2 A common mistake is to use a dose that is too low or to taper the dose too rapidly. For tapering prednisone after prolonged use, ibuprofen or colchicine should be introduced to facilitate the “weaning” process.15 Alternatively, intrapericardial application can avoid systemic side effects and is highly effective.7
Pericardial effusion and cardiac tamponade
Pericardiocentesis is indicated for clinical tamponade, suspicion of purulent or neoplastic pericarditis, or for symptomatic patients, despite medical treatment for more than one week.3 Pericardiocentesis may not be necessary when the effusion is small and resolving with anti-inflammatory treatment. In chronic effusions causing no haemodynamic compromise pericardiocentesis is indicated if additional diagnostic procedures are available (for example, pericardial fluid and tissue analyses, pericardioscopy, and epicardial/pericardial biopsy) to reveal the aetiology of the disease and permit further treatment.6,7,12–14 Aortic dissection is a major contraindication.3 Relative contraindications include uncorrected coagulopathy, anticoagulant treatment, thrombocytopenia < 50000/mm3, and small, posterior, and loculated effusions. In acute traumatic haemopericardium and purulent pericarditis surgical drainage is more appropriate.
Pericardiocentesis can be performed either using fluoroscopic (cardiac catheterisation laboratory) or echocardiographic guidance. ECG monitoring from the puncturing needle is not an adequate safeguard. Right heart catheterisation can be performed simultaneously with pericardiocentesis, allowing haemodynamic monitoring and exclusion of effusive–constrictive disease.16 The subxiphoid approach has been used most commonly, with a long needle with a mandrel (Tuohy or thin walled 18 gauge) directed towards the left shoulder at a 30° angle to the skin. This route is extrapleural and avoids the coronary, pericardial, and internal mammary arteries. The operator intermittently attempts to aspirate fluid and injects small amounts of contrast medium. After aspiration of the fluid the needle must be promptly replaced with a soft J tip guidewire which, after dilatation, is exchanged for a multi-holed pigtail catheter. It is prudent to drain the fluid in stepped amounts of < 1 litre at a time to avoid acute right ventricular dilatation.
Echocardiographic guidance of pericardiocentesis is technically less demanding and can be performed at the bedside.17 After identification of the shortest route to the effusion by echocardiography the pericardium is entered intercostally (usually in the sixth or seventh rib space in the anterior axillary line).
The feasibility of pericardiocentesis is high (93%) in patients with anterior effusion > 10 mm, while the rate of success is only 58% with small, posteriorly located effusions.16 Fluoroscopic and haemodynamic monitoring contribute to a better outcome following the procedure (93.1% v 73.3%) in comparison to emergency pericardial puncture with no imaging control.16 In addition fluoroscopic guidance using the epicardial halo phenomenon (fig 3) significantly increased the feasibility of the procedure in patients with small effusions (200–300 ml; 92.6% v 84.9%) and very small effusions (< 200 ml; 89.3% v 76.7%).18 Echocardiographic guidance improved efficacy to 96% for pericardiocentesis of loculated pericardial effusion after cardiac surgery and to 99% for rescue pericardiocentesis.17
Figure 3.
Fluoroscopic guidance of pericardiocentesis using the epicardial halo phenomenon (black vertical arrows) in lateral angiographic view. The left hand panel shows the puncturing needle (horizontal black arrow) approaching the pericardium. The right hand panel shows angiographic contrast material in the pericardial space (horizontal white arrow) after successful pericardial puncture.
The most serious, but rare, complications are lacerations/perforations of the myocardium and the coronary vessels. In addition, patients can experience air embolism, pneumothorax, arrhythmias (usually vasovagal bradycardia), and puncture of the peritoneal cavity or abdominal viscera.16 Internal mammary artery fistulas, acute pulmonary oedema, and purulent pericarditis have rarely been reported. The safety of pericardiocentesis was significantly improved with the introduction of echocardiographic or fluoroscopic guidance. Recent large echocardiographic series reported an incidence of major complications of 1.3–1.6%.17 In a large series of fluoroscopy guided percutaneous pericardiocenteses, cardiac perforations occurred in 0.9%, serious arrhythmias in 0.6%, arterial bleeding in 1.1%, pneumothorax in 0.6%, infection in 0.3%, and a major vagal reaction in 0.3%.16 Incidence of major complications was also significantly reduced by utilising fluoroscopic guidance and the epicardial halo phenomenon.18
Pericardial constriction
Pericardiectomy is the only therapeutic approach that may remove permanent constriction, by aiming to resect the diseased pericardium as far as possible. The indications for surgery should be based upon clinical symptoms, echocardiography findings, CT/MRI, and heart catheterisation. There are two standard approaches: (1) antero-lateral thoracotomy (fifth intercostal space); and (2) median sternotomy (faster access to the aorta and right atrium if extracorporeal circulation becomes necessary). A primary installation of cardiopulmonary bypass cannot, however, be recommended, due to the enhanced diffuse bleeding following systemic heparinisation. If severe calcified adhesions between the peri- and epicardium are present, or if the epicardium is generally affected (“outer porcelain heart”), surgery carries a high risk of either incomplete success or severe myocardial damage.
An alternative approach in such cases may be “laser shaving” using an Excimer laser. Areas of strong calcification or dense scaring may be left as islands, separated from the other areas, to avoid bleeding. Major complications include acute perioperative cardiac insufficiency (regional bulging or complete dilatation of one or both ventricles) and ventricular wall rupture. Cardiac mortality and morbidity at pericardiectomy is mainly caused by the pre-surgically unrecognised presence of myocardial atrophy or myocardial fibrosis. Exclusion of patients with extensive myocardial fibrosis and/or atrophy led to the reduction of the mortality rate for pericardiectomy from 50% to 5%.5 Left ventricular ejection fraction increases due to improved ventricular filling, but complete normalisation of cardiac haemodynamics after the procedure has been reported in only 60% of patients.2 Postoperative low cardiac output should be treated by fluid substitution and catecholamines, high doses of digitalis, and, in the most severe cases, intra-aortic balloon pump. If the indication for surgery is established early, long term survival after pericardiectomy matches that of an age and sex matched general population.
SPECIFIC TREATMENT MODALITIES
Viral pericarditis
In many patients pericardial viral infection is self limiting and no specific treatment is necessary. In chronic or recurrent symptomatic pericardial effusion and confirmed viral infection the following specific treatment is under investigation:
CMV pericarditis: hyperimmunoglobulin once daily, 4 ml/kg intravenously on days 0, 4, and 8; 2 ml/kg on day 12 and 16
Coxsackie B pericarditis: interferon alpha 2.5 × 106 IU/m2 subcutaneously three times per week
Adenovirus and parvovirus B19 positive myocarditis: immunoglobulin 10 g intravenously on days 1 and 3.3
Bacterial pericarditis
Purulent pericarditis is an absolute indication for pericardial drainage and rinsing of the pericardial cavity, combined with high doses of systemic antibiotic treatment. A combination of antistaphylococcal antibiotic and aminoglycoside, followed by tailored antibiotic treatment according to the results of pericardial fluid and blood cultures, is mandatory.3 Intrapericardial instillation of antibiotics (for example, gentamycin) can be useful but is not sufficient. Frequent irrigation of the pericardial cavity with urokinase or streptokinase, using large catheters, may liquefy the purulent exudate, but open surgical drainage by subxiphoid pericardiotomy is preferable. Pericardiectomy is required in patients with dense adhesions, loculated and thick purulent effusion, recurrence of tamponade following drainage, as well as persistent symptoms or progression to constriction. Perioperative mortality of up to 8% was reported for pericardiectomy, with the total mortality being much higher.2
Tuberculous pericarditis
Tuberculous pericarditis should be promptly treated with a combination of three or four tuberculostatic drugs for 9–12 months, according to standard protocols.9 Prednisone should be administered along with antituberculous drugs in relatively high initial doses (1 mg/kg), maintained for 5–7 days, and progressively reduced until discontinued in 6–8 weeks.9 Corticosteroids reduce the host reaction to mycobacterial infections, minimise exudation, fibrin deposition, and proliferation of tuberculomata, may decrease symptoms and signs, and reduce mortality in tuberculous pericarditis. However, despite combination therapy, some patients develop constriction. If symptoms and signs of constrictive pericarditis remain 6–8 weeks after full tuberculostatic and corticosteroid treatment, pericardiectomy is indicated.
Uraemic pericarditis
Most patients with uraemic pericarditis respond within 1–2 weeks to haemo- or peritoneal dialysis, with resolution of chest pain and reduction of the pericardial effusion. To avoid intrapericardial haemorrhage, heparin-free haemodialysis should be used. Patients with pretamponade should undergo pericardiocentesis before haemodialysis since acute fluid removal with haemodialysis can lead to cardiovascular collapse and tamponade. Peritoneal dialysis, which does not require heparinisation, may be therapeutic in pericarditis resistant to intensified haemodialysis, or if heparin-free haemodialysis cannot be performed. However, it can compromise respiratory function because of intraperitoneal fluid accumulation. NSAIDs and systemic corticosteroids have limited success when intensive dialysis is ineffective. Large, non-resolving symptomatic effusions should be treated with instillation of intrapericardial corticosteroids (triamcinolone) after pericardiocentesis or subxiphoid pericardiotomy.2 Pericardiectomy is indicated only in refractory, severely symptomatic patients.
Post-pericardiotomy syndrome
Treatment of this syndrome is based on administration of an NSAID or colchicine. Preventive application of colchicine before cardiac surgery has also been investigated, but it did not reveal significant results because of the relatively small sample size.3
Post-infarction pericarditis
Ibuprofen is the agent of choice. Aspirin, up to 650 mg every four hours for 2–5 days, has also been successfully applied. Other non-steroidal agents risk thinning the infarction zone.2 Corticosteroid treatment is used for refractory symptoms but can delay infarction healing.
Haemopericardium in aortic dissection
In patients with aortic dissection with involvement of the ascending aorta (DeBakey type I or II and Stanford type A) pericardiocentesis is contraindicated, because of the risk of intensified bleeding.3 Surgery should be performed immediately after the diagnosis is established by echocardiography and/or CT/MRI (in tamponade even if no coronary angiography or aortography is available).
Neoplastic pericarditis
There are no randomised trials comparing the efficacy and safety of different systemic and/or intrapericardial therapeutic modalities in neoplastic pericardial disease. Systemic antineoplastic treatment as baseline therapy and pericardiocentesis to relieve symptoms, establish diagnosis, and enable intrapericardial instillation of cytostatic/sclerosing agents is the common approach. Prevention of recurrences, observed in 40–70% of patients with large malignant pericardial effusion, may be achieved by intrapericardial instillation of sclerosing agents, cytotoxic drugs, immunomodulators, systemic antitumour therapy, percutaneous balloon pericardiotomy, radiation therapy or surgical methods.2,3,19,20 Treatment tailored to the histology and type of tumour in the course of lung (adenocarcinoma) and breast cancer therapy indicate that intrapericardial administration of cisplatin is effective in 83–93% of cases, with no major side effects during the follow up.19 Intrapericardial instillation of thiotepa was effective in 83–89% of cases, together with a favourable safety profile.20
Tetracycline as a sclerosing agent controls malignant pericardial effusion in around 85% of cases, but side effects and complications were reported quite frequently: fever (19%), chest pain (20%), and atrial arrhythmias (10%).3 Although classic sclerotherapy after intrapericardial instillation of tetracycline, doxycycline, minocycline, and bleomycin is an effective procedure, constrictive pericarditis secondary to fibrosis remains a severe problem in long term survivors. Other antineoplastic drugs (5-fluorouracil and aclarubicine) or immunomodulators (interferon, interleukin-2, OK-432) have been tested only in very small numbers of patients. Radiation therapy is very effective (93%) in controlling malignant pericardial effusion in patients with radiosensitive tumours such as lymphomas and leukemias. Although intrapericardial administration of radionuclides has yielded very good results, it is not widely accepted because of the logistical problems associated with their radioactivity.3 A percutaneous balloon pericardial window creates a pleuropericardial direct communication, which allows fluid to be drained into the pleural space. In patients with large malignant pericardial effusions and recurrent cardiac tamponade, it seems to be effective (90–97%) and relatively safe but has the potential risk of disseminating neoplastic cells.2 Pericardiectomy is rarely indicated, and mainly used for treating pericardial constriction or complications of previous procedures.
Management of chylopericardium
Chylopericardium after thoracic or cardiac surgery is preferably treated by pericardiocentesis and diet. In some cases, after initial drainage the disorder may be resolved through dietary management (medium chain triglycerides) alone.2 If further production of chylous effusion continues, surgical treatment is mandatory. When conservative treatment and pericardiocentesis fail, pericardio-peritoneal shunting by means of a pericardial window is a reasonable option. Alternatively, when the course of the thoracic duct has been precisely identified, ligation and resection of the thoracic duct just above the diaphragm has proved to be the most effective treatment.
Supplementary Material
REFERENCES
- 1.Maisch B, Ristić AD. The classification of pericardial disease in the age of modern medicine. Curr Cardiol Rep 2002;4:13–21. ▸ A new classification of pericardial diseases based on pericardioscopy, pericardial/epicardial biopsy, and molecular and immunological analyses of pericardial effusion and tissue is presented. [DOI] [PubMed] [Google Scholar]
- 2.Spodick DH. Pericardial diseases. In: Braunwald E, Zipes DP, Libby P, eds. Heart disease, 6th ed. Philadelphia: WB Saunders, 2001:1823–76. ▸ An excellent, comprehensive textbook chapter covering all aspects of pericardial diseases.
- 3.Maisch B, Adler Y, Erbel R, et al. Scientific statement of the European Society of Cardiology: diagnosis and management of pericardial diseases. Eur Heart J (in press). ▸ First official document of the European Society of Cardiology making management stratification based on the levels of evidence and classes of indication for the diagnosis and treatment of pericardial diseases.
- 4.Merce J, Sagrista-Sauleda J, Permanyer-Miralda G, et al. Should pericardial drainage be performed routinely in patients who have a large pericardial effusion without tamponade? Am J Med 1998;105:106–9. ▸ One of a series of excellent contributions from Professor Soler-Soler’s group on clinical aspects of pericardial effusion and cardiac tamponade. [DOI] [PubMed] [Google Scholar]
- 5.Rienmuller R, Gurgan M, Erdmann E, et al. CT and MR evaluation of pericardial constriction: a new diagnostic and therapeutic concept. J Thorac Imaging 1993;8:108–21. [DOI] [PubMed] [Google Scholar]
- 6.Maisch B, Bethge C, Drude L, et al. Pericardioscopy and epicardial biopsy: new diagnostic tools in pericardial and perimyocardial diseases. Eur Heart J 1994;15(suppl C):68–73. ▸ One of the first studies on flexible percutaneous pericardioscopy, revealing the role of molecular methods and immunology in the diagnostic assessment of patients. [DOI] [PubMed] [Google Scholar]
- 7.Maisch B, Ristić AD, Pankuweit S. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone: the way to avoid side effects of systemic corticosteroid therapy. Eur Heart J 2002;23:1503–8. ▸ First clinical study on autoreactive pericarditis and intrapericardial treatment with triamcinolone, showing high efficacy and low incidence of side effects during the follow up. [DOI] [PubMed] [Google Scholar]
- 8.Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest 1997;111:1213–21. [DOI] [PubMed] [Google Scholar]
- 9.Hakim JG, Ternouth I, Mushangi E, et al. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart 2000;84:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 2003;361:1168–73. [DOI] [PubMed] [Google Scholar]
- 11.Koh KK, Kim EJ, Cho CH, et al. Adenosine deaminase and carcinoembryonic antigen in pericardial effusion diagnosis, especially in suspected tuberculous pericarditis. Circulation 1994;89:2728–35. [DOI] [PubMed] [Google Scholar]
- 12.Nugue O, Millaire A, Porte H et al. Pericardioscopy in the etiologic diagnosis of pericardial effusion in 141 consecutive patients. Circulation 1996;94:1635–41. [DOI] [PubMed] [Google Scholar]
- 13.Porte HL, Janecki-Delebecq TJ, Finzi L, et al. Pericardioscopy for primary management of pericardial effusion in cancer patients. Eur J Cardiothorac Surg 1999;16:287–91. [DOI] [PubMed] [Google Scholar]
- 14.Seferović PM, Ristić AD, Maksimović R, et al. Diagnostic value of pericardial biopsy: improvement with extensive sampling enabled by pericardioscopy. Circulation 2003;107:978–83. ▸ Recent study on pericardial biopsy revealing the contribution of endoscopic guidance to the diagnostic value of the procedure. [DOI] [PubMed] [Google Scholar]
- 15.Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation 1998;97:2183–5. ▸ Multicentre study on recurrent pericarditis revealing the high efficacy of colchicine treatment. [DOI] [PubMed] [Google Scholar]
- 16.Seferović PM, Ristić AD, Maksimović R, et al. Therapeutic pericardiocentesis: up-to-date review of indications, efficacy, and risks. In: Seferović PM, Spodick DH, Maisch B, eds, Maksimović R, Ristić AD, associate eds. Pericardiology: contemporary answers to continuing challenges. Belgrade: Science, 2000:417–26.
- 17.Tsang TS, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc 2002;77:429–36. ▸ The latest of several excellent contributions from the Mayo Clinic on the application of echocardiography for guidance of pericardiocentesis, revealing the high feasibility and excellent safety of the procedure. [DOI] [PubMed] [Google Scholar]
- 18.Maisch B, Ristić AD. Tangential approach to small pericardial effusions under fluoroscopic guidance in the lateral view: the halo phenomenon [abstract]. Circulation 2001;103(suppl A):II–730. [Google Scholar]
- 19.Maisch B, Ristić AD, Pankuweit S, et al. Neoplastic pericardial effusion: efficacy and safety of intrapericardial treatment with cisplatin. Eur Heart J 2002;23:1625–31. ▸ Study on intrapericardial treatment of neoplastic pericardial effusion revealing higher efficacy of cisplatin in lung cancer than in breast cancer patients [DOI] [PubMed] [Google Scholar]
- 20.Colleoni M, Martinelli G, Beretta F, et al. Intracavitary chemotherapy with thiotepa in malignant pericardial effusion: an active and well tolerated regimen. J Clin Oncol 1998;16:2371–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.