Abstract
Objectives: To investigate myocardial blood flow of the morphological right systemic ventricle in unoperated patients with congenitally corrected transposition of the great arteries (CCTGA) by positron emission tomography (PET).
Design: Prospective cross sectional clinical study.
Setting: Tertiary referral centre for paediatric cardiology.
Patients: 15 patients with CCTGA were investigated by PET with nitrogen-13 ammonia at rest and during adenosine vasodilatation. A subgroup of seven patients had isolated CCTGA (group A, mean (SD) age 30.3 (11.9) years) and the remaining eight patients had complex CCTGA associated with subpulmonary stenosis; four of this second group also had ventricular septal defect (group B, mean (SD) age 30.6 (16.4) years). Eleven healthy adults (mean (SD) age 26.2 (5.1) years) served as the control group.
Results: Resting myocardial blood flow was not different between both groups of patients with CCTGA and the controls. Hyperaemic blood flows were significantly lower in both groups of CCTGA than in the control group (mean (SD) 195 (21) ml/100 g/min in group A, 201 (27) ml/100 g/min in group B, 309 (74) ml/100 g/min in the control group; p < 0.001). Thus, coronary flow reserve was significantly lower in both groups of CCTGA than in the control group (mean (SD) 2.5 (0.28) in group A, 2.6 (0.48) in group B, and 4.0 (0.73) in the control group; p < 0.001).
Conclusion: Blood flow measurements suggest that coronary reserve is decreased in the absence of ischaemic symptoms in patients with CCTGA. The global impairment of stress flow dynamics may indicate altered global vasoreactivity, and quantitative changes in microcirculation suggest that their role in the pathogenesis of systemic right ventricular dysfunction is important.
Keywords: congenitally corrected transposition, myocardial blood flow, systemic right ventricle, positron emission tomography
Congenitally corrected transposition of the great arteries (CCTGA), first described by Karl von Rokitansky in 1875,1 is a rare condition in which there is both atrioventricular and ventriculoarterial discordance. The systemic ventricle is of right morphology and patients are at high risk of developing systemic ventricular dysfunction.2–4 The clinical course is complicated by associated intracardiac defects such as ventricular septal defect, subvalvar and valvar pulmonary stenosis, left atrioventricular valve regurgitation, and atrioventricular conduction disturbances.5 The pathophysiology of cardiac systemic ventricular failure is unknown. The inability to perform adequately as a systemic ventricle may result from the macroscopic and microscopic structural features of the morphologically right ventricular myocardium.6,7 One of the compensatory mechanisms to chronic pressure overload is an increase in myocardial mass—that is, cardiac hypertrophy and an increased demand on the coronary arterial oxygen supply, which is mainly from a morphological right coronary system, with a mirror image distribution.8 It may be possible that inadequate coronary perfusion limits the extent to which the heart can compensate for the pressure overload and thus may precipitate a decline in ventricular function. Recent studies by Hornung and colleagues9 showed that patients with unoperated CCTGA have had a high prevalence of myocardial perfusion defects with consecutive regional wall motion abnormalities and impaired ventricular contractility. In this study, positron emission tomography (PET) with nitrogen-13 ammonia at rest and during pharmacological vasodilatation with adenosine was used for the first time to assess quantitative alterations of myocardial blood flow (MBF) and vasoreactivity in unoperated patients with CCTGA.
METHODS
Patients
Fifteen patients (six female and nine male patients, mean (SD) age 30.6 (19.4) years, range 6–59 years) with a history of CCTGA without operation were investigated. All patients received extensive information about the aim of the study and agreed to participate. All gathered data and technical investigations were part of clinical follow up. All patients, parents, or legal guardians gave written informed consent before PET.
For analysis of MBF and haemodynamic data, the 15 patients were divided into two subgroups.
Group A consisted of seven patients (mean (SD) age 30.3 (11.9) years, range 14–48 years) with isolated CCTGA and without associated structural cardiac abnormalities. Five patients were in sinus rhythm, one had atrioventricular dissociation in the form of a junctional escape rhythm, and one patient had sequential atrioventricular pacing because of congenital complete heart block. Six patients were in New York Heart Association (NYHA) functional class I and one was in class II. One patient (age 48 years) was treated with angiotensin converting enzyme inhibitors.
Group B compromised the remaining eight patients (mean (SD) age 30.6 (16.4) years, range 6–59 years) in whom associated anomalies were present. All patients had moderate subpulmonary stenosis. Four patients additionally had a ventricular septal defect. No patient had signs of pulmonary hypertension. Six patients were in sinus rhythm and two patients had sequential atrioventricular pacing because of complete heart block. Five patients were in NYHA class I, two in class II, and one in class III. Three patients were treated with angiotensin converting enzyme inhibitors. Under resting conditions all patients had normal oxygen saturation.
Echocardiography was performed by a single experienced operator, blinded to all other results, by the transthoracic approach using a Vingmed system five (General Electrics, Horten, Norway. Because of the cardiac anatomy and the upright position of the interventricular septum, we used a qualitative, subjective assessment of systemic ventricular function from multiview two dimensional echocardiography, grading it as normal or mildly, moderately, or severely impaired. Atrioventricular valve regurgitation was graded semiquantitatively by Doppler colour flow mapping and was graded as trivial, mild, moderate, and severely insufficient.10
All patients were exercised on a bicycle using a ramp protocol, starting at 15 W and followed by an increase of 20 W/min.
MBF was measured by PET as part of a routine clinical follow up. At the time of inclusion, all patients were normally developed and asymptomatic. None of the patients had electrocardiographic signs or symptoms of stress induced ischaemia during maximal exercise testing.
Eleven healthy young adults (mean (SD) age 26.2 (5.1) years, range 21–35 years) formed a control group. They had no evidence of cardiovascular disease on the basis of the absence of symptoms and risk factors, normal resting ECG, and normal exercise test. In this study systemic right ventricles in patients with CCTGA were being compared with systemic left ventricles in healthy control subjects.
This group had already been used as a control group in a previously published study.11
Positron emission tomography
MBF was quantified non-invasively at rest and during adenosine induced vasodilatation12) by dynamic PET with 13N ammonia. Images were acquired using an ECAT EXACT or an ECAT 951 scanner (Siemens/CTI, Knoxville, Tennessee, USA). After positioning the patient, a transmission scan was acquired for correction of photon attenuation. Subsequently, 13N ammonia (approximately 0.3 mCi/kg) was injected intravenously at rest and a dynamic sequence of 21 frames was acquired over 20 minutes. After 50 minutes to allow for decay of 13N ammonia, adenosine (0.14 mg/g/min) was infused continuously over five minutes. Two minutes after the onset of adenosine infusion, a second dose of 13N ammonia was administered and a dynamic imaging sequence similar to the rest study was started. Heart rate, blood pressure, and a 12 lead ECG were monitored continuously throughout the procedure. MBF at rest and during hyperaemia were quantified using a volumetric sampling approach and a validated three compartment model, which is also applicable for patients with CCTGA and morphological right systemic ventricles.13,14
Because of the relation between MBF at rest and the rate–pressure product as an index of cardiac work,15 resting flow was normalised to the corresponding rate–pressure product. In addition to quantification of global MBF, regional myocardial perfusion was analysed visually. Summed images of tracer distribution in the last three frames of the dynamic sequence were interpreted for the presence of reversible or persistent defects.
To obtain an index of coronary vascular resistance, mean aortic blood pressure as a measure of coronary perfusion pressure was divided by blood flow values at rest and during adenosine infusion.
Statistical analysis
Mean and standard deviation were calculated for all continuous variables. Differences between the groups were tested for significance by one way analysis of variance and the post hoc test (least square difference). Changes from baseline to adenosine stress were compared by the paired Student’s t test. Univariate analysis of the effects of each continuous variable was performed with linear regression. All tests of significance were two tailed and p < 0.05 was considered to be significant.
RESULTS
Echocardiography
In isolated CCTGA (group A) the estimated ventricular function of the morphological right systemic ventricle was normal in all patients. Four patients had mild and three patients had moderate regurgitation of the systemic atrioventricular valve (morphological tricuspid valve). In group B ventricular function was normal in six and moderately reduced in two patients, who additionally had a ventricular septal defect. The mean (SD) gradient across the subpulmonary stenosis was 63.8 (9.9) mm Hg. All patients had significant hypertrophy of the subpulmonary ventricle. Regurgitation of the systemic atrioventricular valve was graded as mild in five, moderate in two, and severe in one patient.
No patient from group A or group B had wall motion abnormalities.
Haemodynamics
Table 1 lists the haemodynamic findings of both groups of patients and the healthy young adults at rest and during adenosine infusion. In all three groups a significant increase of heart rate and rate–pressure product during adenosine infusion was found. At rest and during hyperaemia, the haemodynamic parameters systolic, diastolic, and mean aortic blood pressure were not significantly different within the groups.
Table 1.
Haemodynamic findings
| CCTGA isolated group A (n=7) | CCTGA + anomalies group B (n=8) | Control group (n=11) | ||||
| Rest | Adenosine | Rest | Adenosine | Rest | Adenosine | |
| Heart rate (beats/min) | 65 (7) | 97 (20)* | 72 (21) | 96 (16)* | 68 (10) | 107 (13)* |
| Systolic BP (mm Hg) | 118 (8) | 119 (9) | 127 (25) | 131 (28) | 121 (16) | 121 (17) |
| Diastolic BP (mm Hg) | 72 (11) | 69 (12) | 79 (18) | 82 (14) | 67 (6) | 64 (6) |
| Mean aortic BP (mm Hg) | 96 (9) | 97 (15) | 103 (21) | 107 (19) | 93 (10) | 92 (10) |
| Rate-pressure product | 7705 (1120) | 11 574 (2925)* | 8988 (2647) | 12 207 (3447)* | 8192 (672) | 12 832 (2230)* |
Data are mean (SD).
*p<0.05 v rest.
BP, blood pressure; CCTGA, congenital corrected transposition of the great arteries.
Myocardial blood flow
At rest, MBF before and after normalisation to the corresponding rate–pressure product did not differ in both groups of patients with CCTGA and healthy young adults (table 2).
Table 2.
Quantitative results of positron emission tomography with nitrogen-13 ammonia
| CCTGA isolated group A (n=7) | CCTGA + anomalies group B (n=8) | Control group (n=11) | |
| Age (years) | 30.3 (11.9) | 30.6 (16.4) | 26.2 (5.2) |
| MBFrest (ml/100g/min) | 76 (9) | 82 (28) | 71 (21) |
| MBFrest normalised (ml/100 g/min) | 78 (17) | 72 (9) | 75 (15) |
| MBFadenosine (ml/100 g/min) | 195 (21)* | 201 (27)* | 309 (74) |
| Coronary flow reserve | 2.5 (0.28)* | 2.6 (0.48)* | 4.0 (0.73) |
| MCRrest (mm Hg/ml/g/min) | 125.5 (16.8) | 141.3 (59.9) | 141.6 (24.8) |
| MCRadenosine (mm Hg/ml/g/min) | 50.4 (9.4)* | 57.7 (21.3)* | 36.6 (6.4) |
Data are mean (SD).
*p<0.001 v healthy young adults.
MBF, myocardial blood flow; MCR, minimal coronary vascular resistance.
Adenosine induced vasodilatation resulted in significantly increased MBF in all three groups (mean (SD) 195 (21) v 78 (17) ml/100 g/min at rest in group A, p < 0.001; 201 (27) v 72 (9) ml/100 g/min in group B, p < 0.001; and 309 (74) v 75 (15) in healthy volunteers, p < 0.001). Hyperaemic blood flow, however, was significantly lower in both groups of patients with CCTGA than in the control group (p < 0.001).
As a result of lower MBF during adenosine infusion, coronary flow reserve (CFR) was greatly attenuated in both groups of patients with CCTGA compared with that in healthy young adults (mean (SD) 2.5 (0.28) in group A, 2.6 (0.48) in group B, and 4.0 (0.73) in healthy volunteers; p < 0.001).
Myocardial flow parameters did not differ between patients with isolated and complex forms of CCTGA and those with mild or severe tricuspid insufficiency.
In CCTGA hyperaemic blood flow and CFR were negatively correlated with age (p < 0.05).
CFR was significantly reduced in patients with echocardiographically reduced systolic ventricular function compared with those with normal function (2.3 (0.16) v 2.8 (0.40); p < 0.005).
Myocardial flow parameters did not differ significantly between patients given angiotensin converting enzyme inhibitors and those who were not treated.
We did not find a significant correlation between myocardial flow parameters (MBF at rest and during stress, CFR) and right ventricular rate–pressure product calculated at rest and exercise.
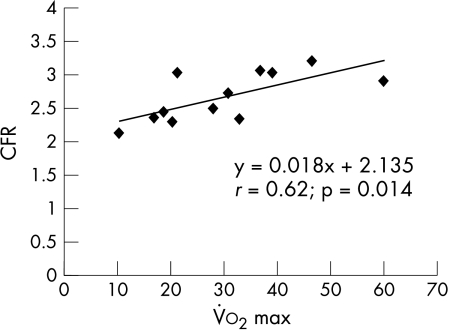
All 15 patients were exercised. From 12 patients gas exchange parameters were available for calculating anaerobic threshold and maximum oxygen consumption (V̇o2max) as a parameter of cardiopulmonary exercise capacity. A significant correlation was calculated between CFR and V̇o2max (r = 0.63; p = 0.014) (fig 1).
Figure 1.
Relation between coronary flow reserve (CFR) and maximum oxygen consumption (V̇o2max).
Heart rate increased significantly in all patients after adenosine administration (group A: rest 65 (7.0) beats/min, adenosine 97.0 (20) beats/min; group B: rest 72.0 (21) beats/min, adenosine 96.0 (16) beats/min; p < 0.05). Those with atrioventricular dissociation developed regular sinus rhythm after adenosine injection.
Myocardial flow parameters did not differ between patients with congenital heart block and sequential atrioventricular pacing, and those with regular sinus rhythm.
Coronary vascular resistance
At rest, coronary vascular resistance was not significantly different between group A, group B, and healthy controls (table 2). Minimal coronary resistance during adenosine vasodilatation was significantly higher in group A and group B than in healthy adults (group A 50.4 (9.4) mm Hg/ml/g/min and group B 57.7 (21.3) mm Hg/ml/g/min v controls 36.6 (6.4) mm Hg/ml/g/min; p < 0.001).
Qualitative analysis of myocardial perfusion
Visual analysis showed an adenosine induced reversible perfusion defect in the apical region of the systemic right ventricle in 1 of 15 patients. This patient was in the subgroup of group B of CCTGA with associated pulmonary stenosis and ventricular septal defect. A stenosis of the epicardial coronary arteries was excluded in that patient by coronary angiography.
DISCUSSION
The present study suggests that adenosine induced MBF and CFR are attenuated in patients with unoperated CCTGA. These alterations seem to be present in patients with isolated CCTGA, as well as in patients with complex forms of CCTGA. Regional abnormalities of myocardial perfusion were detectable by visual analysis in only one patient. However, quantitative MBF during adenosine and CFR were also attenuated in patients without visual abnormalities, suggesting that global vasoreactivity was altered. These perfusion abnormalities may contribute to the impaired ventricular function seen in these patients with CCTGA. Although the patients were clinically well with balanced circulations, a significant correlation was found between CFR and V̇o2max, which highlights the clinical impact of our findings.
Fate of the systemic right ventricle in patients with CCTGA
About half of the patients with CCTGA, especially those without associated defects, do not present until adult life.16 These patients may become symptomatic between the third and fifth decade of life with symptoms of congestive heart failure, decreased functional capacity, varying degrees of atrioventricular block, or sudden death. Some of these patients may also present with symptoms of angina pectoris.17–19
CCTGA is a model in which the anatomical right ventricle functions against a systemic afterload for a long time, on average for 30.6 (16.4) years in the current study, and in which the patients have not been subjected to the confounding variables associated with complex surgical repair. Murray and colleagues20 showed that chronic pressure overload of the morphological right ventricle by pulmonary artery banding in conscious dogs is associated with significant hypertrophy of the right ventricle. Right ventricular hypertrophy is characterised by a substantial increase in blood flow per gram of right ventricular myocardium comparable with myocardial flow dynamics of a normal left ventricle. Several studies in the animal model have shown that coronary hyperaemic response and minimal coronary vascular resistance are similar for the morphological left and hypertrophied systemic right ventricle.21–23 Similar results were obtained in human studies. Therefore, it should be feasible to compare the myocardial flow dynamics of a morphological right systemic ventricle in patients with CCTGA with the flow pattern of a normal left ventricle in healthy young adults.24.
Despite the increase in MBF to the hypertrophied right ventricle, no significant changes in the ratio of capillary to muscle fibre number were observed. Therefore, it appears that increases in blood flow to the hypertrophied right ventricle are most likely a result of increases in right ventricular metabolic requirements.20 These data suggest that the development of right ventricular hypertrophy is characterised by a sustained compensatory response of the coronary circulation to the augmented work load and mass, and that it is not associated with a proliferative response of the vasculature supplying the enlarged ventricle.
Impaired stress flow dynamics
The exact pathogenesis of these findings is unclear and cannot be determined from this study.
Abnormal coronary blood flow regulation caused by microvascular disease or dysfunction causes limited coronary microvasculator dilator reserve and myocardial ischaemia in the absence of large epicardial coronary artery disease. Inadequate dilator capacity and inappropriate or excess constrictor response are two divergent vascular properties that may be an important pathophysiological background.
A series of animal experiments by Bache and Vrobel25 showed that the hypertrophied heart is prone to subendocardial hypoperfusion and myocardial ischaemia during pacing tachycardia and exercise without epicardial coronary disease. Ischaemia is most likely to be caused by an oxygen demand–supply imbalance. Inadequate oxygen supply may result from decreased effective coronary driving pressure caused by impaired systemic ventricular (right ventricle) diastolic function and increased wall stress caused by the intrinsic geometry of the right systemic ventricle with increased end diastolic volume.26
Wall stress is an important determinant of myocardial oxygen consumption, myocardial contractile state, and diastolic function.27 The rise in systolic wall stress, the measure of afterload most closely related to systolic function, results in decreased ventricular performance. High wall stress has been associated with a less favourable prognosis because of electrical instability and myocardial hypoperfusion.27
Thus, not only is the oxygen supply reduced (decreased CFR) but at the same time oxygen demand is enhanced (increased wall stress). One can hypothesise that this mismatch between supply and demand may lead to the occurrence of potential subendocardial ischaemia and cardiac failure.
Coronary capillaries have a crucial role in the transport of oxygen and nutrients to the myocardium. Although the overall control of coronary blood flow is a function mainly of the small arteries and arterioles, capillary density and distribution in space greatly influence the exchange process between blood and tissue.28 These transport mechanisms are critical for normal cardiac function, and changes associated with cardiac hypertrophy in either one may contribute to the impaired function of the hypertrophic heart.29
The typical coronary distribution of corrected transposition is that of coronary artery–ventricular concordance. The morphological right ventricle, which is on the left side of the heart, is supplied by a morphological right coronary artery system.8 The systolic–diastolic flow of a normal right coronary artery system changes to a phasic coronary flow pattern with a sharp reduction in systolic right coronary artery flow, which closely resembles the phasic coronary artery flow in the normal left ventricle. In combination with a sparse coronary artery supply to the right ventricle, shown by Hornung and colleagues9 during cardiac catheterisation, this system may provide inadequate flow in the presence of the considerable myocardial hyperplasia and hypertrophy seen when the right ventricle functions at systemic pressures for long periods. This observation was also made by Lowensohn and colleagues30 while examining the effects of congenital pulmonary artery stenosis on right coronary artery flow in conscious dogs.
Masden and Franch,31 in a review of isolated CCTGA, noted that no patient younger than 35 years had symptoms of congestive heart failure. This clinical observation is supported by findings in our patient population. All our patients younger than 35 years had normal systolic ventricular function and were symptom-free. In our study right ventricular function tended to be attenuated in the older subjects, consistent with a reduction of hyperaemic MBF after adenosine administration and impaired CFR. We did not find the high incidence of perfusion defects described by Hornung and colleagues.9 Nevertheless, altered global vasoreactivity and quantitative changes in microcirculation suggest that they have an important role in the pathogenesis of right ventricular dysfunction in this congenital cardiac lesion, especially in older patients.
The findings of our study are comparable with those involving patients with transposition of the great arteries late after atrial switch operation.32 As unoperated patients with CCTGA are a natural model of a morphological right systemic ventricle, patients after Mustard or Senning operation had open heart surgery, which additionally affects MBF parameters in a negative way; nevertheless, attenuation of the coronary microcirculation cannot be explained only by the insult of open heart surgery. In both groups of patients the precise cause of perfusion abnormalities is unknown, but the possibility of recurrent asymptomatic ischaemia caused by impaired MBF may lead to subendocardial fibrosis and development of late ventricular dysfunction.
Study limitations
This study was performed at a tertiary care centre for congenital cardiac disease. Looking at patients of a tertiary referral centre may result in a negative selection bias, as traditionally patients with more severe lesions are referred and followed up there. Thus, the sample of patients may not represent the typical population of CCTGA seen by general practitioners or cardiologists.
Conclusion
This study suggests that the problem of reduced ventricular function of the morphological right systemic ventricle in patients with CCTGA is caused by a mismatch between oxygen demand and supply. The intrinsic geometry of the morphological right systemic ventricle and the process of remodelling of the ventricular myocardium with myocyte hypertrophy, interstitial fibrosis, and inadequate capillary growth out of proportion to increased right ventricular mass may contribute to limited nutritional support with consequent ventricular deterioration.
Abbreviations
CCTGA, congenitally corrected transposition of the great arteries
CFR, coronary flow reserve
MBF, myocardial blood flow
NYHA, New York Heart Association
V̇o2max, maximum oxygen consumption
REFERENCES
- 1.Von Rokitansky CF. Die Defekte der Scheidewände des Herzens. Vienna: Wilhelm Braunmueller, 1875.
- 2.Sano T, Riesenfeld T, Karl TR, et al. Intermediate-term outcome after intracardiac repair of associated cardiac defects in patients with atrioventricular and ventriculoarterial discordance. Circulation 1995;92(suppl 9):II272–8. [DOI] [PubMed] [Google Scholar]
- 3.Graham TP, Parrish MD, Boucek RJ, et al. Assessment of ventricular size and function in congenitally corrected transposition of the great arteries. Am J Cardiol 1983;51:244–51. [DOI] [PubMed] [Google Scholar]
- 4.Webb GD, McLaughlin PR, Gow RM, et al. Transposition complexes. Cardiol Clin 1993;11:651–64. [PubMed] [Google Scholar]
- 5.Ikeda U, Furuse M, Suzuki O, et al. Long term survival in aged patients with corrected transposition of the great arteries. Chest 1992;101:1382–5. [DOI] [PubMed] [Google Scholar]
- 6.Murphy JH, Barlai-Kovach MM, Mathews RA, et al. Rest and exercise right and left ventricular function late after the mustard operation: assessment by radionuclide ventriculography. Am J Cardiol 1983;51:1520–5. [DOI] [PubMed] [Google Scholar]
- 7.Allwork SP, Bentall HH, Becker AE, et al. Congenitally corrected transposition of the great arteries: morphologic study of 32 cases. Am J Cardiol 1976;38:910–23. [DOI] [PubMed] [Google Scholar]
- 8.Losekoot TG, Becker AE. Discordant atrioventricular connection and congenitally corrected ttransposition. In: Anderson RH, Macartney FJ, Shinebourne EA, et al, eds. Paediatric cardiology. Edinburgh: Churchill Livingstone, 1987:867–87.
- 9.Hornung TS, Bernard EJ, Jaeggi ET, et al. Myocardial perfusion defects and associated ventricular dysfunction in congenitally corrected transposition of the great arteries. Heart 1998;80:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyatake K, Izumi S, Okamoto M, et al. Semiquantitative grading of severity of mitral regurgitation by real-time two-dimensional flow imaging technique. J Am Coll Cardiol 1986;7:82–8. [DOI] [PubMed] [Google Scholar]
- 11.Hauser M, Bengel FM, Kuehn A, et al. Myocardial blood flow and flow reserve after coronary reimplantation in patients after arterial switch and Ross operation. Circulation 2001;103:1875–80. [DOI] [PubMed] [Google Scholar]
- 12.Wilson RF, Wyche K, Christensen BV, et al. Effects of adenosine on human coronary arterial circulation. Circulation 1990;82:1595–606. [DOI] [PubMed] [Google Scholar]
- 13.Hutchins GD, Schwaiger M, Rosenspire KV, et al. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomography imaging. J Am Coll Cardiol 1990;15:1032–42. [DOI] [PubMed] [Google Scholar]
- 14.Krivokapich J, Smith GT, Huang SC, et al. Nitrogen-13-ammonia myocardial imaging at rest and with exercise in normal volunteers: quantification of absolute myocardial perfusion with dynamic positron emission tomography. Circulation 1989;80:1328–37. [DOI] [PubMed] [Google Scholar]
- 15.Czernin J, Muller P, Chan S, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 1993;88:62–9. [DOI] [PubMed] [Google Scholar]
- 16.Presbitero P, Somerville J, Rabajoli F, et al. Corrected transposition of the great arteries without associated defects in adult patients: clinical profile and follow up. Br Heart J 1995;74:57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajwa N, Bianco JA, Stone CK. Thallium myocardial scintigraphy in congenitally-corrected transposition of the great arteries. J Nucl Med 1991;32:1611–3. [PubMed] [Google Scholar]
- 18.Friedberg DZ, Nadas AS. Clinical profile of patients with congenitally corrected transposition of the great arteries. N Engl J Med 1979;282:1053–9. [DOI] [PubMed] [Google Scholar]
- 19.Dodek A, Neill WA. Corrected transposition of the great arteries masquerading as coronary artery disease. Am J Cardiol 1972;30:910–3. [DOI] [PubMed] [Google Scholar]
- 20.Murray PA, Baig H, Fishbein MC, et al. Effects of experimental right ventricular hypertrophy on myocardial blood flow in conscious dogs.J Clin Invest 1979;64:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray PA, Vatner SF. Abnormal coronary vascular response to exercise in dogs with severe right ventricular hypertrophy. J Clin Invest 1981;67:1314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray PA, Vatner SF. Reduction of maximal coronary vasodilator capacity in conscious dogs with severe right ventricular hypertrophy. Circ Res 1981;48:27–33. [DOI] [PubMed] [Google Scholar]
- 23.Mueller TM, Marcus ML, Kerber RE, et al. Effect of renal hypertension and left ventricular hypertrophy on the coronary circulation of dogs. Circ Res 1978;42:543–9. [DOI] [PubMed] [Google Scholar]
- 24.Doty DB, Wright CB, Hiratzka LF, et al. Coronary reserve in volume-induced right ventricular hypertrophy from atrial septal defect. Am J Cardiol 1984;54:1059–63. [DOI] [PubMed] [Google Scholar]
- 25.Bache RJ, Vrobel TR. Effects of exercise on blood flow in the hypertrophied heart. Am J Cardiol 1978;44:1029. [DOI] [PubMed] [Google Scholar]
- 26.Parrish MD, Graham TP, Bender HW, et al. Radionuclide angiographic evaluation of right and left ventricular function during exercise after repair of transposition of the great arteries. Circulation 1983;67:178–83. [DOI] [PubMed] [Google Scholar]
- 27.Sugishata Y, Iida K, Ohtsuka S, et al. Ventricular wall stress revisited: a keystone of cardiology. Jpn Heart J 1994;35:577–87. [DOI] [PubMed] [Google Scholar]
- 28.Turek Z, Hoofd L, Rakusan K. Myocardial capillaries and tissue oxygenation. Can J Cardiol 1986;2:98–103. [PubMed] [Google Scholar]
- 29.Anversa P, Sonnenblick EH. Ischemic cardiomyopathy: pathophysiologic mechanisms. Prog Cardiovasc Dis 1990;32:1–22. [DOI] [PubMed] [Google Scholar]
- 30.Loewensohn HS, Khouri EM, Gregg DE, et al. Phasic right coronary artery blood flow in conscious dogs with normal and elevated right ventricular pressures. Circ Res 1976;39:760–6. [DOI] [PubMed] [Google Scholar]
- 31.Masden RR, Franch HH. Isolated congenitally corrected transposition of the great arteries. In: Hurst JW, ed. Update III: the heart. New York: McGraw Hill, 1980:59–83.
- 32.Singh TP, Humes RA, Muzik O, et al. Myocardial flow reserve in patients with a systemic ventricle after atrial switch repair. J Am Coll Cardiol 2001;37:2120–5. [DOI] [PubMed] [Google Scholar]