Despite progress with new drugs to treat pulmonary arterial hypertension (PAH), a proportion of patients still deteriorate despite medical treatment. Atrial septostomy is emerging as an effective palliative treatment associated with notable improvement in symptoms, exercise capacity, and survival. Experience of this approach in the UK is limited and many cardiologists are either unaware of this option or regard it as an experimental procedure. We report the results of the first 12 atrial septostomy procedures performed at our centre in nine patients to palliate severe PAH.
PATIENTS AND METHODS
Between December 1999 and December 2001 nine female patients underwent atrial septostomy. Three patients had PAH associated with scleroderma; six had primary pulmonary hypertension (PPH). The symptom leading to consideration of septostomy was exercise syncope in five patients and pre-syncope in the others. Six patients were New York Heart Association (NYHA) functional class IV; the remainder were functional class III.
All patients underwent right heart catheter assessment 0–8 months before septostomy. The catheter data are shown in table 1 together with the probability of survival to two years calculated using the regression equation from the US National Institutes of Health (NIH) primary pulmonary hypertension registry.1
Table 1.
Characteristics and result of the first nine patients undergoing atrial septostomy at Freeman Hospital
| No. | Sex | Age | Diagnosis | NYHA class | Symptoms | MVo2 (%) | RAP (mmHg) | Cardiac index (l/min/m2) | mPAP (mmHg) | PVR index (WU.m2) | NIH probability of surviving 2 years | 02 saturation before | 02 saturation after | Size of largest balloon | Procedure | Syncope relieved | Repeat procedure |
| 1 | F | 33.5 | PPH | III | Pre-syncope | NA | 13 | 1.56 | 67 | 37.2 | 0.45 | 94 | 89 | 15 | Uneventful | Yes | At 15 months hole reopened |
| 2 | F | 57.0 | SSc | IV | Syncope | 60.2 | 16 | 1.34 | 59 | 37.2 | 0.42 | 93 | 91 | 18 | Uneventful | Yes | No |
| 3 | F | 72.3 | PPH | IV | Pre-syncope | NA | 24 | 1.51 | 50 | 24.6 | 0.37 | 95 | 87 | 15 | Uneventful | Yes | At 4 months hole enlarged to 20 mm |
| 4 | F | 68.8 | PPH | IV | Syncope | 67 | 12 | 1.76 | 56 | 28.4 | 0.50 | 97 | 78 | 12 | Thyrotoxic at time of procedure | Yes | No |
| 5 | F | 25.9 | PPH | III | Syncope | 65 | 5 | 1.82 | 51 | 24.7 | 0.61 | 99 | 85 | 12 | BP dropped for procedure. Transfused 2 units | Yes | At 3 months hole enlarged to 18 mm |
| 6 | F | 24.5 | PPH | III | Pre-syncope | 55 | 8 | 1.43 | 53 | 34.2 | 0.53 | 97 | 84 | 18 | Uneventful | Yes | No |
| 7 | F | 76.3 | SSc | IV | Pre-syncope | 61 | 7 | 1.49 | 37 | 20.2 | 0.59 | 98 | 93 | 18 | Uneventful | Yes | No |
| 8 | F | 80.2 | SSc | IV | Syncope | 62 | 8 | 1.82 | 55 | 25.8 | 0.55 | 95 | 91 | 18 | Transfused pre-procedure. Uneventful | Yes | No |
| 9 | F | 69.2 | PPH | IV | Syncope | 59 | 6 | 1.44 | 53 | 31.3 | 0.56 | 96 | 87 | 12 | Uneventful | Yes | No |
NIH probability refers to the likelihood of surviving 2 years from the date of the right heart catheter based on the regression equation from D’Alonzo et al1 using haemodynamic parameters.
MVo2, mixed venous oxygen saturation; NA, not available; NYHA, New York Heart Association; mPAP, mean pulmonary artery pressure, PPH, primary pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; SSc, scleroderma.
Atrial septostomy was performed in a standard cardiac catheter laboratory under local anaesthetic. A transeptal puncture was performed using a Brockenbrough needle via a long femoral sheath. A guide wire was passed into the left atrium and lodged in a pulmonary vein followed by serial dilation of the interatrial septum using 9, 12, 15, and 18 mm balloon catheters in a stepwise fashion. After each dilation, systemic oxygen saturation was observed for at least five minutes and the defect was considered adequate when oxygen saturation remained between 80–85%. Following the procedure all patients were observed for at least 12 hours on a high dependency unit before returning to the respiratory ward where they stayed until day 3 following the procedure.
All but one of the patients performed standard six minute walking tests before and after the septostomy.2 Patient 8 was unable to walk at the time of septostomy following a pelvic fracture sustained during a syncopal episode.
RESULTS
Atrial septostomy was carried out successfully in all patients with no procedure related deaths. One patient (patient 5) suffered chest pain and hypotension after the first balloon dilation requiring analgesia and transfusion of packed red cells. A pericardial effusion was seen on echocardiography after the procedure raising the possibility of a pericardial bleed. She was subsequently discharged at day 4 with no further problems. Three patients had repeat atrial septostomy. Patient 3 underwent repeat procedure at four months to enlarge the septal hole. She was haemodynamically fragile during the initial procedure and we took a cautious approach to avoid excessive right to left shunting. Patients 1 and 5 underwent a repeat procedure because of closure of the hole at 15 months and three months, respectively. The patient characteristics are shown in table 1.
Two patients have died since atrial septostomy and one has undergone heart/lung transplantation. Patient 4 was thyrotoxic at the time of septostomy. The procedure was performed without complications but she died six weeks later from a cardiac arrest. In patient 8, septostomy was delayed after she fractured her pelvis following a syncopal fall. The procedure was delayed until judged safe to anticoagulate her. She died two months after septostomy from complications of the pelvis fracture.
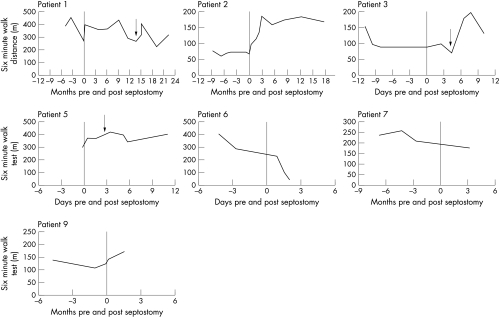
Exercise data were available for seven patients (fig 1). The six minute walking test improved in three patients with benefit accruing progressively over 12 weeks following the procedure. In three patients there was little change in exercise capacity; two of these patients had six minute walking tests of around 400 m before septostomy. At this level, the six minute walking test is a poor discriminator for detecting improvement. In one patient there was continued deterioration in exercise capacity despite septostomy. She was the only patient to report increased dyspnoea associated with the drop in systemic oxygen saturation. Dyspnoea remained a prominent symptom and she elected to be listed for transplantation.
Figure 1.
Exercise capacity graphs for the seven patients in whom exercise data are available. The graphs show the six minute walking distance before and after atrial septostomy (time zero). Patients 1, 3, and 5 had repeat or revision atrial septostomy at time point indicated by the arrow.
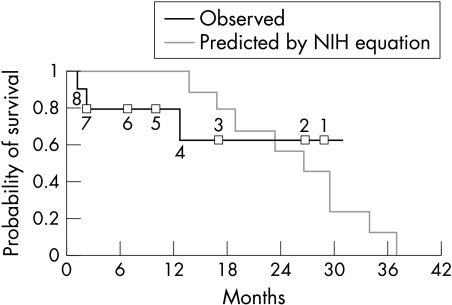
Survival of our patients is calculated to July 2002 and compared to the expected survival based on NIH registry data.1,3 Using the regression equation developed from this dataset, the number of days are calculated to the point where the probability of survival falls below 50%. Invasive haemodyamic data used for calculation of expected survival was carried forward to the date of septostomy. Figure 2 shows the transplant-free survival observed in our patients after septostomy together with the predicted survival calculated using the NIH registry equation.
Figure 2.
Expected survival for each patient may be calculated using the NIH regression equation based on right atrial pressure, mean pulmonary artery pressure, and cardiac index.1 The time until the probability of survival falls to less than 0.5 may thus be calculated. Expected survival from the date of right heart catheter is plotted on the graph together with the observed survival after septostomy in our patients. The numbers on the graph indicate the number of patients at risk at each point.
DISCUSSION
The first atrial septostomy in a patient with PPH was performed in 1983.4 Early series from the USA reported high mortality—largely caused by difficulty in achieving accurate control of the size of the atrial shunt.5–9 Improvements in patient selection together with use of a sequential, balloon dilation technique have improved the safety of the procedure.10–13 Recent series show significant improvements in exercise capacity and survival when compared with retrospective controls.13 However, to date, atrial septostomy has been performed in less than 300 PPH patients worldwide and the total UK experience is limited to about 30 patients.
Why might atrial septostomy confer survival advantage for patients with PAH? The right ventricle receives its blood supply from the aorta. During exercise, systemic vascular resistance falls. In a patient with PPH, cardiac output cannot rise in response to exercise because of fixed obstruction in the pulmonary vascular bed. Systemic blood pressure therefore falls resulting in syncope together with reduced perfusion of the right ventricular myocardium. Peacock and colleagues have shown that right ventricular systolic pressure in patients with PAH rises sharply in response to exercise.14 Twenty five per cent of patients with PPH die from sudden death.1 We speculate that the mechanism of these sudden deaths may include arrhythmias precipitated by acute myocardial ischaemia. Most patients with PAH die from progressive right ventricular failure.1 Repeated ischaemic stress to the right ventricle myocardium may contribute to progressive failure of the right ventricle.
One patient (patient 6) experienced unremitting dyspnoea following atrial septostomy. Her oxygen saturation was no lower than other patients and echocardiography showed that right ventricular dimensions and function both improved. However, the improvement in ventricular function was not translated into an improvement in six minute walking distance. Following this experience we are considering introducing pre-procedure assessment of hypoxic ventilatory response.
The comparison of the observed survival in our patients with the expected survival based on the NIH registry data1 is not ideal. The NIH registry data were collected in the era before widespread use of prostacyclin. The right heart catheter data used to calculate predicted survival was obtained up to seven months before septostomy was performed. Data were carried forward to the date of septostomy in the prediction model and hence may not accurately reflect the haemodynamics at the time of septostomy.
Spontaneous improvement is rare in PAH. Five of our patients were deteriorating at the time of septostomy despite receiving long term prostacyclin treatment. Patients who had improvement and resolution of symptoms with medical treatment were not considered for septostomy. Hence, the time interval between catheter data and septostomy in this series may tend to underestimate the gain from septostomy.
The longest duration of follow up in our series to date is only three years. We think it likely that survival will be improved overall, however, because the previous inexorable decline seen in several patients appears to have been arrested. The true survival benefit of an intervention can only properly be assessed in a randomised controlled study, but PPH is an uncommon and malignant disease. The rarity of the condition makes recruitment for such a trial challenging and the availability of transplantation would make it unethical to run a study with a true survival end point. The arrival of new drugs means that “standard treatment” has changed frequently in recent years. These factors together present substantial problems for designing studies to assess the survival advantage of a new intervention. The recent drug trials in PPH have all used the six minute walk test as the primary end point.15–17 Changes in six minute walk correlate well with survival.3 Most of our patients have shown significant improvement in exercise capacity following septostomy.
Case selection, the level of invasive monitoring required during and after the procedure, adjustment of medications, the role of blood transfusion pre- or post-procedure, and the ideal size of shunt are all areas that remain incompletely defined and clinical judgement remains important. Most reports of atrial septostomy for pulmonary hypertension to date have included only patients with PPH. Palliation is likely to be equally effective in patients with PAH arising in association with systemic sclerosis, as in three of our patients. This patient group is older than patients with PPH and may be less able to cope with complex treatments such as intravenous prostacyclin. Patients with systemic sclerosis, however, may derive substantial benefit from atrial septostomy.18
Selection of patients and management of their care before and after atrial septostomy are both as challenging as the procedure itself. No centre yet has extensive experience with atrial septostomy for pulmonary hypertension but it is a procedure with a high risk of fatal complications. Atrial septostomy should only be attempted in units with wide experience of managing patients with PAH and within the context of familiarity with the other treatment options now available for this fatal disease.
Abbreviations
NIH: National Institutes of Health
NYHA: New York Heart Association
PAH: pulmonary arterial hypertension
PPH: primary pulmonary hypertension
REFERENCES
- 1.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–9. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognstic significance of six minute walk test in patients with primary pulmonary hypertension. Am J Respir Crit Care Med 2000;161:487–92. [DOI] [PubMed] [Google Scholar]
- 4.Rich S, Lam W. Atrial septostomy as palliative therapy for refractory primary pulmonary hypertension. Am J Cardiol 1983;51:1560–1. [DOI] [PubMed] [Google Scholar]
- 5.Rich S, Dodin E, McLaughlin VV. Usefulness of atrial septostomy as a treatment for primary pulmonary hypertension and guidelines for its application. Am J Cardiol 1997;80:369–71. [DOI] [PubMed] [Google Scholar]
- 6.Nihill MR, O’Laughlin MP, Mullins CE. Effects of atrial septostomy in patients with terminal cor pulmonale due to pulmonary vascular disease. Cathet Cardiovasc Diagn 1991;24:166–72. [DOI] [PubMed] [Google Scholar]
- 7.Kerstein D, Levy PS, Hsu DT, et al. Blade balloon atrial septostomy in patients with severe primary pulmonary hypertension. Circulation 1995;91:2028–35. [DOI] [PubMed] [Google Scholar]
- 8.Hayden AM. Balloon atrial septostomy increases cardiac index and may reduce mortality among pulmonary hypertension patients awaiting lung transplantation. J Transpl Coord 1997;7:131–3. [DOI] [PubMed] [Google Scholar]
- 9.Sobrino N, Frutos A, Calvo L, et al. [Palliative interatrial septostomy in severe pulmonary hypertension]. Rev Esp Cardiol 1993;46:125–8. [PubMed] [Google Scholar]
- 10.Hausknecht MJ, Sims RE, Nihill MR, et al. Successful palliation of primary pulmonary hypertension by atrial septostomy. Am J Cardiol 1990;65:1045–6. [DOI] [PubMed] [Google Scholar]
- 11.Takigiku K, Shibata T, Yasui K, et al. Successful blade atrial septostomy in a patient with severe primary pulmonary hypertension – a case report. Jpn Circ J 1997;61:877–81. [DOI] [PubMed] [Google Scholar]
- 12.Thanopoulos BD, Georgakopoulos D, Tsaousis GS, et al. Percutaneous balloon dilatation of the atrial septum: immediate and midterm results. Heart 1996;76:502–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandoval J, Gaspar J, Pulido T, et al. Graded balloon dilation atrial septostomy in severe primary pulmonary hypertension. A therapeutic alternative for patients nonresponsive to vasodilator treatment. J Am Coll Cardiol 1998;32:297–304. [DOI] [PubMed] [Google Scholar]
- 14.Raeside DA, Chalmers G, Clelland J, et al. Pulmonary artery pressure variation in patients with connective tissue disease: 24 hour ambulatory pulmonary artery pressure monitoring. Thorax 1998;53:857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002;165:800–4. [DOI] [PubMed] [Google Scholar]
- 16.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903. [DOI] [PubMed] [Google Scholar]
- 17.Galie N, Humbert M, Vachiery JL, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2002;39:1496–502. [DOI] [PubMed] [Google Scholar]
- 18.Allcock RJ, O’Sullivan JJ, Corris PA. Palliation of systemic sclerosis-associated pulmonary hypertension by atrial septostomy. Arthritis Rheum 2001;44:1660–2. [DOI] [PubMed] [Google Scholar]