Cardiovascular diseases, in particular coronary artery disease (CAD), are the leading cause of death in industrialised countries. Established options for revascularisation include angioplasty and surgical bypass, both of which are not suitable in 20–30% of patients in whom the extent of coronary atherosclerosis is especially severe. An alternative treatment strategy for revascularisation is therefore warranted both to control symptoms as well as to alter the course of advanced CAD. An ideal candidate to fill in this gap is therapeutic promotion of coronary collateral growth—that is, the induction of natural bypasses. In order to reach this goal, a comprehensive understanding of the human coronary collateral circulation with regard to its relevance, accurate assessment, the pathogenetic and pathophysiological aspects, and the different therapeutic options is mandatory.
RELEVANCE OF THE CORONARY COLLATERAL CIRCULATION
The coronary collateral circulation has been recognised for a long time as an alternative source of blood supply to a myocardial area jeopardised by ischaemia. More than 200 years ago, Heberden described a patient who had been nearly cured of his angina pectoris by sawing wood each day,w1 a phenomenon called “warm up” or “first effort angina” which was traditionally ascribed to coronary vasodilation with opening of collateral vessels to support the ischaemic myocardium. Alternatively, and more recently, “walk through angina” has been interpreted as a biochemical (that is, ischaemic preconditioning) rather than a biophysical (that is, collateral recruitment) event leading to heightened tolerance against myocardial ischaemia. Both mechanisms probably contribute to the described phenomenon, which is easily obtainable by careful history taking of the patient.1 Aside from the controversies just alluded to, there have been numerous investigations demonstrating a protective role of well versus poorly grown collateral arteries (fig 1) showing smaller infarcts,w2 less ventricular aneurysm formation, improved ventricular function,w2 fewer future cardiovascular events,2 and improved survival.3
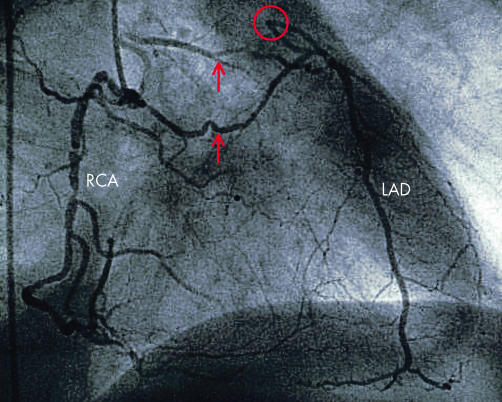
Figure 1.
Coronary angiogram with injection of radiographic contrast medium into the right coronary artery (RCA) and complete filling of the proximally occluded (red circle) left anterior descending coronary artery (LAD) via collateral channels (arrows).
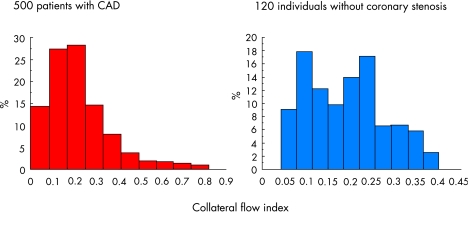
However, the functional relevance of coronary collateral vessels in humans had also been a matter of debate for many years.w3 Much of this controversy was likely the result of inadequate means for gauging human coronary collaterals and the investigation of populations too small to be representative for all the patients with CAD. The latter is well illustrated by the fact that among patients with haemodynamically significant atherosclerotic lesions, only about a third have functionally sufficient coronary collaterals which are able to prevent signs of myocardial ischaemia during brief vascular occlusions (fig 24). In the absence of stenoses, it has been traditionally assumed that coronary arteries are functional end arteries.w4 Using direct and quantitative intracoronary collateral measurements (see below), it has, however, been documented very recently that the notion of the human coronary circulation being built without preformed functioning anastomoses between vascular territories is a myth rather than reality; in the absence of obstructive CAD or even in entirely normal hearts, there has been collateral flow to a briefly occluded coronary artery sufficient to prevent ECG signs of myocardial ischemia in 20–25% of the population studied (fig 2).5
Figure 2.
Frequency distribution (per cent of the entire population, vertical axis) of collateral flow obtained during coronary artery balloon occlusion relative to normal flow during vessel patency (collateral flow index, horizontal axis). The left panel shows the distribution in 500 patients with haemodynamically relevant coronary artery stenoses. In the right panel, the distribution in 120 individuals without coronary stenoses is depicted.
ASSESSMENT
Natural coronary occlusion model (chronic total occlusion model)
In the situation of a spontaneously occurring coronary artery occlusion without myocardial infarction, a well developed collateral circulation must be the reason for the salvaged cardiac muscle. A pathophysiological alternative to this scenario is indicated by the following equation describing infarct size (IS), and it consists of an exceedingly small ischaemic myocardial area at risk (AR)w5: IS = coronary occlusion time × AR × collateral supply−1
The entire filling of a chronically occluded, collateral receiving (that is, ipsilateral) coronary artery from a collateral supplying (that is, contralateral) vessel (fig 1) illustrates that AR is closely and inversely dependent on collateral flow, to the extent that the AR of a certain vessel may disappear in the presence of well grown collaterals. The validity of the concept described in the above equation has been recently confirmed in the clinical setting by documenting that coronary occlusion time no longer plays a role as a predictor for IS in the presence of a collateral relative to normal flow (collateral flow index; see below) ⩾ 25%.6 Thus, to detect normal ventricular wall motion in the presence of a proximal or mid chronic occlusion represents a way of qualifying “good” collateral flow. The major disadvantage of this qualitative method for collateral assessment is that it requires coronary angiography to detect vascular occlusion. Having established the diagnosis of an entirely blocked coronary artery, various myocardial perfusion tracers (different radioactive tracers, echocontrast media) are in principle appropriate to measure the gold standard of collateral function (that is, absolute perfusion in ml/min/g of tissue) non-invasively by positron emission tomography or Doppler echocardiography.w6 w7
Artificial coronary occlusion model (angioplasty model)
At present, invasive cardiac examination is a prerequisite for reliable qualitative or quantitative assessment of coronary collaterals. In the natural occlusion model, it is needed to confirm total vascular obstruction, and in the artificial occlusion model, it is essential for briefly blocking the vessel using an angioplasty balloon catheter. In addition, systematically consistent and exclusive collateral characterisation (fig 3) requires the permanent or temporary occlusion of the epicardial collateral receiving artery, yielding the so called recruitable—as opposed to spontaneously visible—collateral flow. Employing the angioplasty model, there are several qualitative and quantitative methods subsequently described which can be used to characterise the collateral circulation.
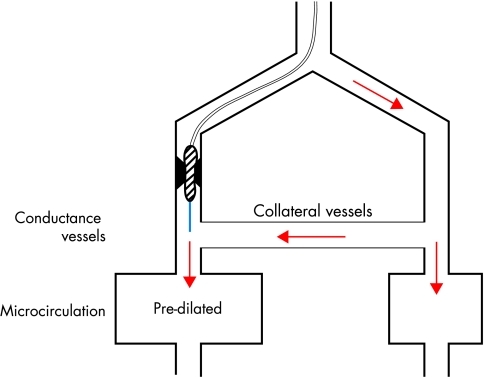
Figure 3.
Diagram illustrating the principle of collateral assessment during coronary artery balloon occlusion using an angioplasty sensor guidewire (blue line). This pressure or Doppler guidewire is positioned distal to the occluded site. Pressure signals (except for venous back pressure) or flow velocity signals detected during vascular occlusion originate from collateral vessels supplying the blocked vascular region.
Angina pectoris and intracoronary ECG during vessel occlusion
The simplest but rather imprecise way to qualify collateral vessels is to ask the patient about the presence of angina pectoris shortly before the end of arterial balloon occlusion. The predictive value of absent or present chest pain for collaterals sufficient or insufficient, respectively, to prevent ischaemia as detected by intracoronary ECG (fig 4) is rather low.7 The use of an intracoronary ECG lead obtained via the angioplasty guidewire for collateral assessment provides a good representation of the pertinent myocardial area. Intracoronary ECG ST segment changes of > 0.1 mV constitute the definition of collaterals insufficient to prevent ischaemia in the respective myocardial territory.7
Figure 4.
Simultaneous recordings of an intracoronary (i.c.) ECG lead (top), phasic (left side) and mean (right side) aortic (Pao, mm Hg), coronary occlusive (Poccl, mm Hg), and central venous pressures (CVP, mm Hg). Pao is gauged via a 6 French coronary artery guiding catheter, Poccl via a pressure guidewire positioned distal of a stenosis to be dilated, and CVP via a right atrial catheter. To the right of the phasic pressure tracings obtained during coronary artery patency, mean pressures are recorded during and after angioplasty balloon deflation. During inflation, there are pronounced ECG ST segment elevations (arrows) indicating collateral vessels insufficient to prevent myocardial ischaemia. Collateral flow index (CFI) is calculated as follows: CFI = (Poccl − CVP)/(Pao − CVP).
Angiographic methods
The coronary angiographic method for collateral qualification most widely used is similar but not identical to the one first described by Rentrop and colleagues.8 The latter provides a score from 0–3 for recruitable collateral vessels upon occlusion of the ipsilateral artery, the former an identical score for spontaneously visible collaterals without artificial vascular occlusion. The score describes epicardial coronary artery filling with radiographic contrast dye via collaterals as follows: 0 = no filling; 1 = small side branches filled; 2 = major side branches of the main vessel filled; 3 = main vessel entirely filled. The fact that in clinical routine, just spontaneously visible collaterals are scored further impairs the method’s sensitivity which is quite blunt already. Recruitable collateral vessel grading in the absence of chronic coronary occlusion, however, requires the insertion of two coronary catheters—that is, one for balloon occlusion of the collateral receiving vessel and the second for injection of contrast dye into the collateral supplying artery. An alternative, semi-quantitative angiographic method consists of determining the number of heart beats during coronary occlusion needed to wash out the angiographic medium injected into the ipsilateral artery immediately before balloon occlusion (that is, washout collaterometry9). The contrast dye caught distal to the occlusive balloon can only be washed out by collateral flow. A washout time of ⩽ 11 heart beats accurately predicts collaterals sufficient to prevent ischaemia during a brief coronary occlusion.
Intracoronary pressure or Doppler sensor measurements
Today, pressure or Doppler sensor-tipped angioplasty guidewires are available which are almost equivalent to regular guidewires in their handling properties. The theoretical basis for the use of intracoronary pressure or blood flow velocity measurements to determine collateral flow relates to the fact that perfusion pressure (> central venous “back” pressure) or velocity signals obtained distal to an occluded stenosis originate from collaterals (fig 3). The measurement of aortic and intracoronary pressure or velocity provides the basic variables for the calculation of a pressure derived or velocity derived collateral flow index (CFI),7 both of which express the amount of flow via collaterals to the vascular region of interest as a fraction of the flow via the normally patent vessel. Pressure derived CFI is determined by simultaneous measurement of mean aortic (Pao), mean distal coronary occlusive (Poccl), and central venous pressure (CVP) (fig 4): CFI = (Poccl − CVP)/(Pao − CVP). Velocity derived CFI is measured by obtaining distal occlusive coronary flow velocity (Voccl) and coronary flow velocity during vessel patency (VØoccl) taken at the same location and following occlusion induced reactive hyperaemia: CFI = Voccl/VØoccl. CFI measurements have been documented to be very accurate with regard to ECG derived dichotomous collateral assessment, with regard to each other, but also to quantitative 99mTc-sestamibi imaging during balloon occlusion.7,10 Pressure and Doppler derived intracoronary collateral measurements are regarded as the reference method for clinical assessment of coronary collateral flow.
PATHOGENESIS
Clinical or “environmental” factors consistently described as influencing the development of coronary collaterals in humans are the severity of coronary artery stenoses,4,8,11 and the duration of myocardial ischaemic symptoms.11 Conversely, there has been discordant information about the influence of metabolic disorders on collateral development such as diabetes mellitus.w8–10 In our experience encompassing 437 non-diabetic and 89 diabetic patients who underwent intracoronary collateral flow measurements, CFI (see above) is practically identical: mean (SD) 0.215 (0.146) and 0.209 (0.128), respectively (p = 0.71). A possible relevance of cholesterol metabolism on the expansion of the collateral circulation has been indicated only experimentally.w11 The presence of systemic hypertension has also been suggested to promote well grown collaterals. Previous studies on the pathogenesis of collateral vessels in humans have often lacked sufficient patient numbers and/or quantitative means for collateral assessment. Although coronary stenosis severity is the independent predictor for collateral development in humans, CFI may vary for a given stenosis of, for example, 95% diameter narrowing between 0.0 and 0.70.4 Conversely, in the absence of a coronary artery stenotic lesion, CFI ranges between 0.05–0.4.5 Therefore, aside from the “environmental” factors just described, the influence of a certain genetic background or the temporarily varying up/down regulation of genes on the formation of well conductive collateral arteries even before the start of CAD must be very relevant, but so far only rarely investigated.w12
FUNCTIONAL ASPECTS
Functional, haemodynamic or biophysical aspects of well grown collateral arteries relate to the fact that they constitute a network within the coronary circulation (fig 3 ). Such connections between adjacent vascular territories together with spatially varying vascular resistances to blood floww13 are the basis for pathophysiological aspects of collaterals rarely considered, such as the redistribution of blood during vasodilation away from a region in need (that is, coronary steal12), the decrease in collateral flow to a certain vascular region following recanalisation of a chronically occluded coronary artery,w14 and the enhanced risk of coronary restenosis following percutaneous coronary intervention in the presence of high and competitive collateral flow to this area.w15 The latter situation is similar to that of competitive flow between a barely stenotic coronary artery and a bypass graft to this vessel which was unnecessarily implanted; usually, the lifespan of such a bypass is very much abridged.
Regarding the vasomotor response of collateral arteries, experimental studies have shown that physical exercise induces a more than twofold perfusion increase in collateral dependent myocardium via β adrenergic and nitric oxide mechanisms.w16 In up to 50% of patients with chronic total coronary artery occlusions, there may be no infarcted myocardium within the vascular territory supplied by the blocked vessel. It is unknown how many such patients remain completely asymptomatic, and therefore must have a normal collateral coronary flow reserve–that is, the capacity to augment flow in response to increased myocardial demand. However, some of the patients with chronic total occlusions without myocardial infarction suffer from chest pain on exertion, and they have been found, using positron emission tomography, to exert a reduced collateral dependent flow reserve in response to dipyridamole.13 Interestingly, the same patients also revealed impaired systolic function in the collateral supplied left ventricular region as opposed to individuals with maintained collateral coronary flow reserve featuring normal regional wall motion.
THERAPEUTIC PROMOTION
An alternative treatment strategy is needed in 20–30% of patients with CAD in whom the extent of coronary atherosclerosis is too severe to allow conventional revascularisation. Therapeutic angiogenesis/arteriogenesis are new strategies for revascularising ischaemic myocardial tissue by formation of “natural bypasses”—that is, collateral vessels. Understanding the many steps and regulatory mechanisms of angio- and arteriogenesis as opposed to vasculogenesis is important for designing such strategies.
Vasculogenesis
The initial steps in the formation of the vascular system during embryonic life involve the differentiation of mesodermal cells into angioblasts that give rise to endothelial cells forming the first primitive blood vessels.14
Abbreviations.
AR: area at risk for myocardial infarction
BFGF: basic fibroblast growth factor
CAD: coronary artery disease
CFI: collateral flow index (no unit)
CVP: central venous pressure (mm Hg)
GM-CSF: granulocyte–macrophage colony stimulating factor
IS: infarct size
Pao: mean aortic pressure (mm Hg)
Poccl: mean coronary occlusive or wedge pressure (mm Hg)
VEGF: vascular endothelial growth factor
Voccl: intracoronary occlusive blood flow velocity (cm/s)
VØoccl: intracoronary non-occlusive blood flow velocity (cm/s)
Angiogenesis
New vessels can subsequently develop from the pre-existing plexus by sprouting and intussusception. This formation of new vessels from pre-existing ones has been called angiogenesis.w17 In addition to endothelial cells, pericytes (for capillaries) and smooth muscle cells (for larger vessels) are necessary for the maturation of these newly growing vessels.w17 Angiogenesis and arteriogenesis are not restricted to the growing organism. Tissue repair and regeneration (for example, wound healing and the cyclic changes of the female reproductive system) are manifestations of angiogenesis. New capillaries form around zones of tissue ischaemia, as occurs in myocardial infarction and stroke. However, vessel formation and growth is also a part of pathogenic processes like proliferative retinopathies, psoriasis, haemangiomas, tumours, and atherosclerotic plaques.15 Upon angiogenic stimulation—for example, after tissue injury or ischaemia with hypoxia and hypoglycaemia—growth factors and inflammatory mediators are released locally leading to vasodilation, enhanced vascular permeability, and accumulation of monocytes and macrophages which in turn secrete more growth factors and inflammatory mediators.w18 These inflammatory cells release metalloproteinases that dissolve the surrounding matrix and the basal membrane of the preformed vessel. Hypoxia sensitises the local endothelial cells to the chemotactic and proliferative effects of various growth factors by upregulating their receptors. Endothelial cells detach from their neighbours, migrate, proliferate, and subsequently form a new vessel with a lumen. Pericytes and smooth muscle cells are also involved in this process.
Relevance and assessment of coronary collaterals: key points.
Well developed coronary collateral arteries in patients with CAD mitigate myocardial infarcts with less ventricular aneurysm formation and improved ventricular function, reduce future cardiovascular events, and improve survival
Collateral arteries preventing myocardial ischaemia during brief vascular occlusion are present in a third of patients with CAD
Among individuals without relevant coronary stenoses, there are preformed collateral arteries preventing myocardial ischaemia in 20–25%
Collateral flow sufficient to prevent myocardial ischaemia during coronary occlusion amounts to ⩾ 25% of the normal flow through the open vessel
Myocardial infarct size is a product of coronary artery occlusion time, area at risk for infarction, and the inverse of collateral supply
Coronary collateral flow can be assessed only during vascular occlusion of the collateral receiving artery
Presently, the gold standard for clinical coronary collateral assessment is the measurement of intracoronary occlusive pressure or velocity derived collateral flow index which expresses collateral flow as a fraction of flow during vessel patency
Arteriogenesis
Cardiologists have long been aware of the occurrence of large and often epicardial collateral vessels after total or subtotal occlusion of a major coronary artery (fig 1). These usually become visible within two weeks following an occlusion, and they arise from preformed arterioles. The remodelling process involved in this recruitment of already existing collateral vessels has been termed arteriogenesis.16 Large bridging collaterals are likely to be much more effective in salvaging ischaemic myocardium at risk for necrosis than small peri-ischaemic capillaries. The complete obstruction of a coronary artery leads to a fall in post-stenotic pressure and to a redistribution of blood to pre-existing arterioles. The resulting stretch and shear forces may lead to an increased expression of certain endothelial chemokines, adhesion molecules, and growth factors. Within days, circulating monocytes attach to the endothelium of the bridging collateral vessels causing a local inflammatory reaction.w18 Matrix dissolution occurs and the vessels undergo a growth process with active proliferation of their endothelial and smooth muscle cells.
Growth factor candidates
A variety of physiological molecules have been identified that appear to promote angio- and arteriogenesis. Most act by stimulating migration and proliferation of endothelial cells and/or smooth muscle cells, like the family of fibroblast growth factors (FGF) and vascular endothelial growth factors (VEGF). Both cause vasodilation by stimulating the release of nitric oxide. It is therefore important in animal as well as clinical studies to differentiate between improved perfusion caused merely by vasodilation and true collateral growth. Other growth factor candidates include placental growth factor, angiopoietin 1, transforming growth factor β, platelet derived growth factor, and about half a dozen other cytokines, proteases, and proteins.15 Arteriogenesis has been shown to be induced by activated macrophages,w19 by lipopolysaccharide,w18 monocyte chemotactic protein-1,16 tumour necrosis factor α, FGF, and also via granulocyte–macrophage colony stimulating factor (recombinant human GM-CSF; Molgramostim).14,17
Pathogenesis and promotion of coronary collaterals: key points.
Clinical variables predicting the development of collateral arteries are the haemodynamic severity of coronary stenoses and the duration of myocardial ischaemic symptoms
25–30% of patients with CAD cannot be revascularised by percutaneous coronary intervention or coronary artery bypass grafting; therapeutic promotion of collateral growth appears to be a valuable treatment strategy in those patients
Promotion of collateral growth should aim at inducing the development of large conductive collateral arteries (that is, arteriogenesis) and not so much the sprouting of capillary-like vessels (that is, angiogenesis)
So far, the largest, controlled clinical angiogenesis trials on the efficacy of VEGF and basic FGF have been negative with regard to treadmill exercise time and myocardial scintigraphic data
Large conductive collateral arteries (that is, arteriogenesis) appear to be effectively promoted via the activation of monocytes/macrophages
Clinical studies
Angiogenesis may be induced by surgical or catheter based delivery of various promotors, such as VEGF and FGF, angiogenic agents most often used in current clinical studies.w20 w21 Although animal studies have established the principle that collateral function improves after delivering angiogenic growth factors,w22 and although the first uncontrolled clinical studies have demonstrated the safety and feasibility of VEGF and basic FGF,w23 efficacy data of angiogenic therapy have been scarce and controversial. The most recent and largest controlled clinical trials using VEGF165 (VIVA: VEGF in ischemia for vascular angiogenesis18) and FGF2 (FIRST: FGF initiating revascularization trial19) in 178 and 337 patients with CAD, respectively, have not shown an effect on the study end points—that is, treadmill exercise time, angina pectoris at 60 and 120 days (VIVA), and, respectively, exercise tolerance test duration at 90 day follow up and changes in the magnitude of myocardial ischaemia by Tc99m SPECT.
Controversy over the ability of angiogenic growth factors to promote coronary collaterals is likely due to the use of end points for their assessment which are too blunt to discern subtle changes in collateral flow. At this early phase of clinical angiogenic/arteriogenic therapy during which screening for the most effective growth factor among more than a dozen candidates has not even started properly, selection of the best agents ought to be based on accurate and direct invasive measurements of coronary collateral flow (figs 3 and 4)—that is, the variable hypothesised to be positively influenced by the growth factors. Equally important, angiogenic factors may have been employed which induce the formation of small, high resistance capillaries (angiogenesis) rather than large interconnecting arterioles (arteriogenesis) which are required for the salvage of myocardium in the presence of occlusive CAD. In the first randomised, placebo controlled clinical trial, GM-CSF has been shown to be effective with regard to sequentially and invasively obtained collateral flow among 21 patients with CAD.20
Arteriogenesis is related to enhanced shear forces at the vessel wall in response to increased flow through pre-existing collateral connections. Therefore, physical exercise would be an ideal therapeutic option for inducing arteriogenesis, because cardiac output and thus coronary flow is elevated along the arterial branches of the coronary circulation during exercise. So far, the only prospective investigation in humans on the effect of exercise regarding collateral growth has employed an insensitive instrument for collateral assessment—that is, angiographic imaging of spontaneously visible collateral vessels—and has been negative.w24 Preliminary data from our own laboratory suggest that even in the absence of CAD, collateral flow, as assessed by intracoronary pressure derived measurements, is augmented substantially in response to endurance exercise training.w25
Supplementary Material
REFERENCES
- 1.Billinger M, Fleisch M, Eberli FR, et al. Is the development of myocardial tolerance to repeated ischemia in humans due to preconditioning or to collateral recruitment? J Am Coll Cardiol 1999;33:1027–35. ▸ A pathophysiological study in 30 patients undergoing coronary angioplasty on the respective contribution of collateral recruitment and ischaemic preconditioning to the development of ischaemic tolerance in response to repetitive coronary occlusions. [DOI] [PubMed] [Google Scholar]
- 2.Billinger M, Kloos P, Eberli F, et al. Physiologically assessed coronary collateral flow and adverse cardiac ischemic events: a follow-up study in 403 patients with coronary artery disease. J Am Coll Cardiol 2002;40:1545–50. [DOI] [PubMed] [Google Scholar]
- 3.Hansen JF. Coronary collateral circulation: clinical significance and influence on survival in patients with coronary artery occlusion. Am Heart J 1989;117:290–5. ▸ The only study of survival rates in patients with CAD and well versus poorly developed coronary collateral vessels as assessed by angiography. [DOI] [PubMed] [Google Scholar]
- 4.Pohl T, Seiler C, Billinger M, et al. Frequency distribution of collateral flow and factors influencing collateral channel development. Functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol 2001;38:1872–8. [DOI] [PubMed] [Google Scholar]
- 5.Wustmann K, Zbinden S, Windecker S, et al. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation 2003;107:2213–20. [DOI] [PubMed] [Google Scholar]
- 6.Lee CW, Park SW, Cho GY, et al. Pressure-derived fractional collateral blood flow: a primary determinant of left ventricular recovery after reperfused acute myocardial infarction. J Am Coll Cardiol 2000;35:949–55. ▸ An elegant study proving the concept of the investigation by Reimer and colleaguesw5 in 70 patients with acute myocardial infarction undergoing primary angioplasty and pressure derived collateral flow index (CFI) measurement. Left ventricular recovery was primarily dependent on CFI, and time to reperfusion was not related to wall motion recovery in patients with CFI ⩾ 0.24. [DOI] [PubMed] [Google Scholar]
- 7.Seiler C, Fleisch M, Garachemani A, et al. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol 1998;32:1272–9. [DOI] [PubMed] [Google Scholar]
- 8.Rentrop KP, Cohen M, Blanke H, et al. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5:587–92. ▸ First description of coronary angiographic grading obtained during balloon occlusion for characterising collateral vessel filling. In 16 patients undergoing coronary angioplasty, collateral filling to the artery being dilated was visualised by contrast injection into the contralateral artery using a second arterial catheter. [DOI] [PubMed] [Google Scholar]
- 9.Seiler C, Billinger M, Fleisch M, et al. Washout collaterometry: a new method of assessing collaterals using angiographic contrast clearance during coronary occlusion. Heart 2001;86:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo H, Watanabe S, Kadosaki T, et al. Validation of collateral fractional flow reserve by myocardial perfusion imaging. Circulation 2002;105:1060–5. ▸ Twenty four consecutive patients with single left anterior descending coronary artery stenosis underwent pressure derived CFI measurement. It was compared with the extent and severity of the defect during coronary occlusion using (99m)Tc-sestamibi imaging at balloon inflation of the respective coronary artery. The authors concluded that pressure derived CFI is highly correlated with the extent and severity of the defect at myocardial perfusion of the territory of the occluded artery. [DOI] [PubMed] [Google Scholar]
- 11.Piek JJ, Koolen JJ, Hoedemaker G, et al. Severity of single-vessel coronary arterial stenosis and duration of angina as determinants of recruitable collateral vessels during balloon occlusion. Am J Cardiol 1991;67:13–17. [DOI] [PubMed] [Google Scholar]
- 12.Seiler C, Fleisch M, Meier B. Direct intracoronary evidence of collateral steal in humans. Circulation 1997;96:4261–7. [DOI] [PubMed] [Google Scholar]
- 13.Vanoverschelde JLJ, Wijns W, Depré C, et al. Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of non-infarcted collateral-dependent myocardium. Circulation 1993;87:1513–23. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Med 2000;6:389–95. ▸ In this review, the cellular and molecular mechanisms underlying the formation of endothelium lined channels (angiogenesis) and their maturation via recruitment of smooth muscle cells (arteriogenesis) during physiological and pathological conditions are summarised, along with possible therapeutic applications. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med 1995;1:27–31. ▸ Review on the concept of the switch to the angiogenic phenotype in tumorigenesis as a net balance of positive and negative regulators of blood vessel growth. The extent to which the negative regulators are decreased during this switch may dictate whether a primary tumour grows rapidly or slowly and whether metastases grow at all. [DOI] [PubMed] [Google Scholar]
- 16.Ito WD, Arras M, Winkler B, et al. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res 1997;80:829–37. ▸ Twelve rabbits were designated to receive monocyte chemotactic protein-1 (MCP-1), placebo, or no infusion. Seven days after femoral artery occlusion, collateral and peripheral conductances were measured and found to be significantly elevated in animals with MCP-1 treatment compared with the control group. In this study, the term “arteriogenesis” was introduced. [DOI] [PubMed] [Google Scholar]
- 17.Buschmann I, Hoefer I, van Royen N, et al. GM-CSF: a strong arteriogenic factor acting by amplification of monocyte function. Atherosclerosis 2001;159:343–56. [DOI] [PubMed] [Google Scholar]
- 18.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial. Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 2003;107:1359–65. [DOI] [PubMed] [Google Scholar]
- 19.Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 2002;105:788–93. [DOI] [PubMed] [Google Scholar]
- 20.Seiler C, Pohl T, Wustmann K, et al. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation 2001;104:2012–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.