Abstract
Objective: To investigate the possible link between the G20210A prothrombin gene variant and different forms of ischaemic heart disease.
Design: Phenotype-specific meta-analysis of 19 studies published within March 2002, globally including 4944 patients and 7090 controls. Sample size, inclusion criteria, geographical location, clinical presentation, age, cardiovascular risk factors, and angiographic extent of disease were extracted from each study. Analyses were done according to Mantel-Haenszel.
Results: Overall, the odds ratio (OR) for unspecified ischaemic heart disease associated with the 20210A allele was 1.21 (95% confidence interval (CI) 0.99 to 1.59, n = 12 034). Similar findings were seen for acute coronary syndromes (unstable angina and myocardial infarction) and for myocardial infarction without age limits (OR 1.24, 95% CI 0.98 to 1.63, n = 10 240; and OR 1.19, 95% CI 0.93 to 1.58, n = 9765). The effects were similar in male and female subjects. In the 1931 subjects < 55 years of age, the OR for myocardial infarction increased to 1.77 (95% CI 1.16 to 3.42) and in the 1359 subjects < 45 years to 2.30 (95% CI 1.27 to 4.59). No significant association was found between the 20210A allele and the presence of angiographically documented coronary disease (OR 1.08, 95% CI 0.70 to 1.64, n = 3444). However, patients with 0/1 vessel disease at angiography showed a greater prevalence of the A allele than those with multivessel disease (relative risk 2.0, 95% CI 1.2 to 3.1, n = 2376).
Conclusions: G20210A prothrombin gene polymorphism may represent a modest but significant risk factor for myocardial infarction at young ages and favour the expression of ischaemic heart disease among individuals who have a limited extent of coronary atherosclerosis at angiography.
Keywords: gene polymorphism, prothrombin 20210A, ischaemic heart disease, meta-analysis
Studies that associate single gene polymorphisms with complex diseases have come under recent criticism. Genetic and phenotypic heterogeneity within the populations studied, together with methodological difficulties (mainly insufficient numbers of subjects), are seen as major confounding factors.1 Meta-analyses may partially overcome these limitations. They represent an acknowledged method of synthesising information from multiple reports2 and are increasingly used in the field of cardiovascular genetics.
Discovered by Poort and colleagues in 1996,3 the single base substitution (G to A at locus 20210) within the 3′-untranslated region of the prothrombin gene is associated with higher plasma concentrations of prothrombin3–5 and currently represents the second most frequent genetic risk factor for venous thromboembolism.6 Its possible role in the pathogenesis of ischaemic heart disease has been investigated in a growing number of clinical studies of different size and design which have yielded apparently conflicting and inconclusive results. We therefore conducted a meta-analysis of the published studies that have linked the G20210A variant to ischaemic heart disease. Our specific aim was to assess the possible association between this gene variant and various predefined phenotypes of ischaemic heart disease.
METHODS
Search strategy and data abstraction
Studies published within March 2002 on G20210A polymorphism were identified by Medline (http://www.ncbi.nlm.nih.gov) entering the words “prothrombin” or “factor II” and “polymorphism” or “variant”. Those comparing the prevalence of the polymorphism in patients with ischaemic heart disease and in healthy controls were selected. No restriction was made according to language. The following information was abstracted from each study: size, criteria for case selection, geographical location, clinical phenotype of cases and controls, age, sex, and potential interacting variables (for example, other cardiovascular risk factors and the extent of disease at angiography).
Identified studies and subgroup analyses
Twenty studies were identified.4,5,7–24 One,24 conducted in black subjects, found a 0% prevalence of the gene variant in both cases and controls and was excluded because of the impossibility of calculating the odds ratio. Prespecified subgroup analyses were conducted according to the following phenotypes:
myocardial infarction without age limits
acute coronary syndrome (unstable angina or myocardial infarction)
myocardial infarction <55 years and <45 years
sex
angiographically documented coronary artery disease.
Statistical methods
Because the rare homozygosity for the 20210 A allele was found in only four of 12 034 subjects, the risk associated with the two mutated genotypes (GA + AA) was compared with the risk associated with GG homozygosity. For each study the crude odds ratio and 95% confidence interval (CI) was calculated using raw data. We used χ2 analysis to assess heterogeneity among studies. These were then pooled according to Mantel-Haenszel.25
RESULTS
Study characteristics
There was one nested prospective13 and 18 cross sectional case–control studies. Their general characteristics are shown in table 1. Most studies enrolled white people of both sexes (male subjects representing the majority in most), but some considered only men, and one enrolled only women. The mean age varied considerably across studies, some restricting the analysis to those under 45, and others using no age limit up to 85 years. The criteria for case selection also differed, ranging from any myocardial infarction or acute coronary syndrome, to a first episode of myocardial infarction or unstable angina, to angiographically confirmed coronary artery disease (table 1). Cases and controls were well matched for sex, race, and geographical origin. With respect to age, the control subjects ranged from healthy newborn infants to adults who were age matched with cases and in whom coronary artery disease was excluded by exercise testing (table 1). In agreement with previous reports,26 healthy North Americans showed a greater prevalence of GA heterozygosity than Europeans (3.4% v 2.4%, p < 0.001), and southern Europeans a greater prevalence than northern Europeans (3.4% v 2.1%, p < 0.001).
Table 1.
General characteristics of the 19 case–control studies on G20210A and ischaemic heart disease
| Authors | Country | Design | Sex | Age (years) | Case selection | *Controls | Cases with A allele | Controls with A allele |
| Rosendaal et al7 | USA | Cross sectional | F | 18 to 44 | First MI | HS, random digit phone | 4/79 | 6/381 |
| Ridker et al13 | USA | Nested prospective | M | 40 to 84 | First MI | HS, age and smoking status matched | 12/404 | 69/1774 |
| Feng et al18 | USA | Cross sectional | Both | 53 (6)† | MI or UA | Non-cardiac patients | 6/100 | 1/25 |
| Arruda et al10 | Brazil | Cross sectional | Both | <82 | MI | Consecutive newborns | 7/220 | 2/293 |
| Doggen et al11 | Netherlands | Cross sectional | M | <70 | First MI | HS, age matched | 10/560 | 8/646 |
| Franco et al5 | Netherlands | Cross sectional | Both | 21 to 50 | CAD at angiography | HS, race matched | 7/263 | 4/400 |
| Croft et al16 | England | Cross sectional | Both | <75 | MI | HS, visitors to non-cardiac patients | 11/539 | 14/498 |
| Prohaska et al15 | Germany | Cross sectional | Both | <85 | CAD at angiography | HS | 6/284 | 7/340 |
| Watzke et al9 | Austria | Cross sectional | Both | 50 (10)† | CAD at angiography | Healthy newborns | 5/98 | 2/102 |
| Ferraresi et al4 | Italy | Cross sectional | Both | <77 | CAD at angiography | HS, age matched | 3/90 | 7/169 |
| Ardissino et al19 | Italy | Cross sectional | Both | <45 | First MI | HS, age matched, negative ET | 11/200 | 8/200 |
| Burzotta et al21 | Italy | Cross sectional | Both | <65 | First MI or UA | HS, age matched | 16/247 | 7/247 |
| Corral et al8 | Spain | Cross sectional | Both | <85 | MI or UA | HS, age matched | 4/101 | 2/101 |
| Vargas et al14 | Spain | Cross sectional | M | <50 | CAD at angiography | HS | 7/175 | 7/200 |
| Redondo et al20 | Switzerland | Cross sectional | Both | 32 to 74 | MI | HS, age matched | 1/177 | 4/85 |
| Inbal et al17 | Israel | Cross sectional | M | <52 | First MI | HS, with negative ET | 7/112 | 6/187 |
| Araújo et al22 | Portugal | Cross sectional | Both | <82 | MI or UA | HS (blood donors) | 5/52 | 2/100 |
| Coulet et al23 | N Ireland, France | Cross sectional | M | 25 to 64 | MI | HS, age matched | 19/599 | 19/663 |
| Eikelboom et al12 | Australia | Cross sectional | Both | <51 | CAD at angiography | HS, randomly selected from the electoral roll | 19/644 | 19/679 |
*Cases and controls were well matched for sex and geographical origin.
†Mean (SD).
CAD, coronary artery disease; ET, exercise test. F, female; HS, healthy subjects; M, male; MI, myocardial infarction; UA, unstable angina.
G20210A and risk of ischaemic heart disease
The 19 eligible studies included a total of 4944 cases and 7090 controls. There was no significant heterogeneity among these studies (χ2 = 25.83, p > 0.1). The prevalence of the mutated genotypes ranged from 0.7–4.7% among controls (mean 2.8%), and from 0.6–6.5% among cases (mean 3.2%). In 14 of the 19 studies, the A allele was more common among cases than controls (not always achieving a statistically significant difference) and less common in five (table 1). The overall odds ratio for unspecified ischaemic heart disease associated with GA+AA in comparison with the GG genotype was 1.21 (95% CI 0.99 to 1.58) (fig 1).
Figure 1.
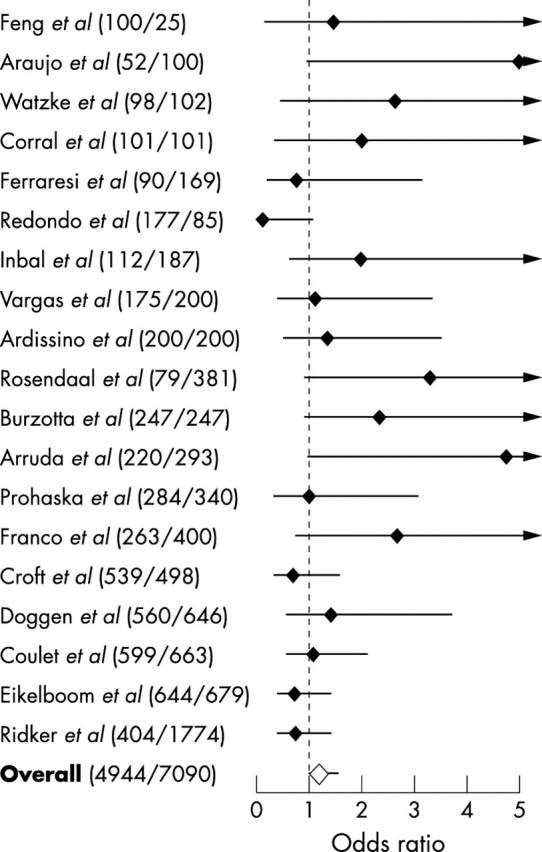
Case–control studies of G20210A and ischaemic heart disease. The odds ratios compare GA + AA with GG genotype in cases and controls. Black diamonds indicate crude odds ratio. The combined odds ratio is indicated by the white diamond. Horizontal lines give the 95% confidence intervals. The numbers of cases and controls are given in parentheses. Studies are ordered according to sample size.
G20210A and risk of myocardial infarction or acute coronary syndrome
In nine reports, the index event for case selection was myocardial infarction.7,10,11,13,16,17,19,20,23 In four additional studies, data in the subgroups of patients with myocardial infarction were available.5,12,18,21 Altogether, these 13 studies included 3687 cases of myocardial infarction and 6078 controls; heterogeneity among them was significant (χ2 = 23.12, 0.025 < p < 0.05). The odds ratio for myocardial infarction associated with the mutated genotypes compared with GG was 1.19 (95% CI 0.93 to 1.58) (fig 2). A similar odds ratio of 1.24 (95% CI 0.98 to 1.63) was obtained by analysing the 15 reports of 3965 cases and 6275 controls that considered acute coronary syndromes (myocardial infarction or unstable angina) (χ2 = 26.59, 0.025 < p < 0.05) (fig 2).
Figure 2.
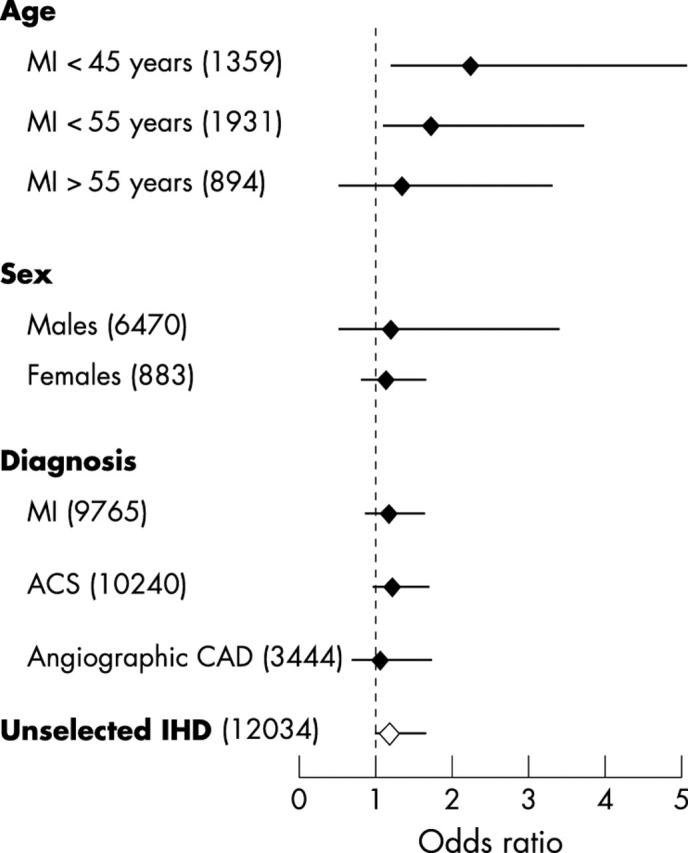
Odds ratios for different ischaemic heart disease phenotypes associated with carrying the 20210A allele. Black diamonds indicate odds ratios (comparing GA+AA with GG genotype of cases and controls) in different subgroups (divided by age, sex, and diagnosis used for case selection). The white diamond indicates the overall odds ratio. Horizontal lines show the 95% confidence intervals. Numbers of cases and controls are given in parentheses. ACS, acute coronary syndromes (myocardial infarction or unstable angina); angiographic CAD, significant coronary artery disease at angiography; IHD, ischaemic heart disease; MI, myocardial infarction.
G20210A and risk of myocardial infarction before 55 or 45 years of age
Six reports (on 624 patients and 1307 controls) enrolled cases with myocardial infarction before the age of 55 or provided data in subjects younger than 55 years.7,10,16,17,19,21 No significant heterogeneity was found across these studies (χ2 = 7.25, p > 0.1). The mean frequency of the GA genotype was 4.7% in cases and 2.2% in controls, with an associated odds ratio for myocardial infarction of 1.77 (95% CI 1.16 to 3.42) (fig 2). Conversely, raw data on subjects older than 55 years were available in two studies only,16,21 (homogeneity χ2 = 1.94, p > 0.1); the GA genotype was present in 17 of 499 cases (3.4%) and in 10 of 395 controls (2.5%), with an associated odds ratio for myocardial infarction beyond 55 years of age of 1.40 (95% CI 0.59 to 2.99).
Four reports on 394 cases and 965 age matched controls considered patients with a first myocardial infarct who were ⩽ 45 years of age.7,10,19,21 These studies did not show significant heterogeneity (χ2 = 3.09, p > 0.1). The frequency of the GA genotype ranged from 4.8–6.7% among cases (mean 5.5%) and from 0.7–2.2% in the corresponding control group (mean 1.9%). The resulting odds ratio associated with the GA genotype compared with the GG was 2.30 (95% CI 1.27 to 4.59) (fig 2).
G20210A and risk of ischaemic heart disease according to sex
Three studies on myocardial infarction and unstable angina were conducted on female subjects7 or provided data regarding female sex.16,21 No significant heterogeneity was found across these studies (χ2 = 3.48, p > 0.1). The mean frequency of the GA genotype was 3.2% in the 279 cases and 2.3% in the 604 controls, with a resulting odds ratio for myocardial infarction among female subjects of 1.23 (95% CI 0.54 to 3.42). Five studies on ischaemic heart disease were conducted on male subjects only11,13,14,17,23 and two further studies provided data relating to male sex16,21 (χ2 = 6.22, p > 0.1). The mean frequency of the GA genotype was 3.1% in the 2452 cases and 3.0% in the 4018 controls, with an associated odds ratio of 1.17 (95% CI 0.85 to 1.61).
G20210A and presence of angiographically documented disease
In six reports,4,5,9,12,14,15 case selection was based on the angiographic documentation of coronary artery disease, irrespective of clinical presentation. No significant heterogeneity was found among these studies (χ2 = 4.58, p > 0.1). The mean frequency of the GA genotype was 2.8% among the 1554 cases and 2.6% among the 1890 controls, with a pooled odds ratio of 1.08 (95% CI 0.70 to 1.64) (fig 2).
G20210A and angiographic extent of disease
The angiographic extent of coronary artery disease was reported in four studies,12,21,27,28 two of which were not case–control and were therefore not included in the present meta-analysis.27,28 Of these four studies, two enrolled subjects undergoing angiography irrespective of clinical presentation (excluding, in one study,12 cases with stenoses of < 50%); they reported no significant difference in genotype distribution according to number of stenosed vessels.12,27 In contrast, the other two studies that selected patients for unstable angina or myocardial infarction (regardless of angiographic findings) found a greater prevalence of the 20210 A allele among patients with no or one vessel disease, compared with those with multivessel disease.21,28 A pooled analysis of the two studies21,27 which provided genotype frequencies in patients with no, one, two, or three vessel disease showed a greater prevalence of the GA genotype among patients with no or one vessel disease than in those with multivessel disease (4.4% v 2.2%; relative risk 2.0, 95% CI 1.2 to 3.1).29
G20210A and cardiovascular risk factors
Of the available studies, 115,8–10,14–16,18,20,22,23 did not provide genotype data in relation to cardiovascular risk factors (apart from age), whereas six7,11–13,17,19 provided general results but not in sufficient detail to undertake subgroup analyses. Of these, three7,11,17 reported a significantly increased risk of myocardial infarction associated with the 20210A allele among smokers or among those with obesity, diabetes, hypertension, or hypercholesterolaemia compared with controls, while three12,13,19 reported no significant interactions. One study,21 specifically aimed at stratifying genotype according to risk factors, found a greater prevalence of the 20210A allele in the subgroup of patients with acute coronary syndrome and no acquired or metabolic risk factor in comparison with healthy controls. Thus further data will be necessary to clarify this issue.
DISCUSSION
Scope of the present meta-analysis
Within the complex scenario of single nucleotide polymorphisms, the G20210A prothrombin variant may be a plausible risk factor for arterial thrombosis, as it may shift the haemostatic balance towards thrombosis through increased plasma prothrombin concentrations.3–5 Many individual reports relating this variant to ischaemic heart disease have, however, produced contrasting results. Discrepant data are not uncommon for studies associating single nucleotide polymorphisms with multifactorial polygenic diseases,1 and sample size and type may be relevant issues. For this reason we did a meta-analysis of available case–control studies on over 12 000 subjects. Two previous reviews analysing the role of the G20210A polymorphism as a risk factor for ischaemic heart disease considered, respectively, a total of 5607 and 5637 individuals (cases plus controls).30,31 To our knowledge, the present analysis is the second largest sample analysed to date for the association between a gene polymorphism and ischaemic heart disease (after that by Di Castelnuovo et al for the PlA1/A2 glycoprotein IIIa gene variant).32
G20210A and unspecified ischaemic heart disease
From the pooled data of all 19 studies (irrespective of age, case selection, and stable or unstable clinical presentation), the 20210A allele was not significantly associated with ischaemic heart disease (odds ratio 1.21). Data from six reports indicated no association between this polymorphism and the presence of angiographically confirmed coronary artery disease (odds ratio 1.08). In unselected populations, the GA+AA genotypes were also found not to be significantly associated with myocardial infarction or acute coronary syndromes, in agreement with a previous meta-analysis which considered 1926 patients (using very strict diagnostic criteria for myocardial infarction) and 3711 controls.31 Similar non-significant odds ratios were found in female and male subjects.
G20210A and myocardial infarction before 55 or 45 years of age
A major limitation of current genetic studies, applied to multifactorial clinical syndromes such as ischaemic heart disease, is the lack of phenotypic markers identifying patient subgroups who may have a different prevalence of specific genetic or environmental causal factors. Heterogeneity in the clinical phenotype of cases may introduce an important bias and prevent the recognition of specific pathogenic components. For this reason, in the present analysis we systematically reviewed various possible clinical markers and found that age younger than 55 or 45 years identified subgroups of patients with myocardial infarction who had an increased prevalence of the G20210A genetic component. Such patients form a group in which the search for new genetic risk factors is expected to be more promising—on the one hand, the traditional environmental cardiovascular risk factors are less often present or have had less time to exert their detrimental effects; on the other hand, the earlier manifestation of disease may be the effect of a stronger genetic predisposition.
We were able to abstract data from about 2000 cases and controls below 55 years of age and from over 1300 subjects below 45 years of age. In five of the six studies of subjects under 55 years of age and in all the four studies of those less than 45 years of age, the prevalence of the 20210 A allele was higher among cases than among controls, resulting in a significantly increased pooled estimate of risk of 1.77 for myocardial infarction at < 55 years, and 2.30 at < 45 years. Such increases in risk are in keeping with a previous report that considered only three studies on subjects younger than 55 years31 and are among the greatest to have emerged from meta-analyses of single nucleotide polymorphisms in the setting of ischaemic heart disease.
G20210A and angiographic extent of disease
Subjects with myocardial infarction at a young age often have limited atherosclerotic involvement.33 Recent data21,28,29 suggest a significant association between the G20210A variant and acute coronary syndromes that occur in patients with limited coronary atherosclerosis, in whom the thrombotic component may be expected to be greater. This signal may become diluted in studies with broad case selection criteria or in those that exclude cases with angiographically non-significant coronary artery disease. However, further evaluations are needed, as such estimations stem from the pooling of small studies which may have undergone a publication bias in favour of positive results.
Study limitations
As for most meta-analyses, the results of the present study may be influenced by publication bias, whereby negative results—especially from small studies—are more likely to remain unpublished. We also cannot exclude the potential for a survival bias, as cases of fatal myocardial infarction were understandably excluded from most studies; a single necropsy study34 of 33 cases of fatal acute myocardial infarction and 165 controls showed an important trend towards an increased prevalence of the GA genotype among cases with myocardial infarction (odds ratio 7.0, 95% CI 0.6 to 82).
Significant heterogeneity emerged for the overall group of studies on myocardial infarction and on acute coronary syndromes. While this may raise questions about the validity of the estimated odds ratio (which, however, was not significant), it also supports the concept that, within the multiple pathogenic mechanisms of coronary thrombosis, the G20210A prothrombin variant may play a heterogeneous role, ranging from non-existent to significant. If this is the case, then owing to the limited sensitivity of the homogeneity χ2 test, there is the theoretical possibility that the other analyses presented may also suffer from heterogeneity.
Conclusions
We report a comprehensive meta-analysis of 19 studies on the association of the prothrombin G20210A polymorphism and ischaemic heart disease. The pooled estimates on 12 034 subjects indicate that the 20210 A allele does not substantially enhance the risk of unspecified ischaemic heart disease, but may increase the risk of developing a myocardial infarct before the age of 55 or 45 years. The available data also suggest that this variant may favour the expression of ischaemic heart disease among patients with angiographically limited coronary atherosclerosis. The search for phenotypic markers (such as age and the extent of coronary artery disease) may represent an important general prerequisite for future genetic studies to produce clinically useful information in this field.35
Acknowledgments
We thank Dr Paolo Burzotta for invaluable assistance with the statistical analyses. FB is recipient of an ESC fellowship in coronary thrombosis.
REFERENCES
- 1.Gambaro G, Anglani F, D’Angelo A. Association studies of genetic polymorphisms and complex disease. Lancet 2000;355:308–11. [DOI] [PubMed] [Google Scholar]
- 2.Gu C, Province MA, Rao DC. Meta-analysis for model-free methods. Adv Genet 2001;42:255–72. [DOI] [PubMed] [Google Scholar]
- 3.Poort SR, Rosendaal FR, Reitsma PH, et al. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996;88:3698–703. [PubMed] [Google Scholar]
- 4.Ferraresi P, Marchetti G, Legnani C, et al. The heterozygous 20210 G/A prothrombin genotype is associated with early venous thrombosis in inherited thrombophilias and is not increased in frequency in arterial disease. Arterioscler Thromb Vasc Biol 1997;17:2418–22. [DOI] [PubMed] [Google Scholar]
- 5.Franco RF, Trip MD, ten Cate H, et al. The 20210 G→A mutation in the 3′-untranslated region of the prothrombin gene and the risk for arterial thrombotic disease. Br J Haematol 1999;104:50–4. [DOI] [PubMed] [Google Scholar]
- 6.Bertina RM. The prothrombin 20210 G to A variation and thrombosis. Curr Opin Hematol 1998;5:339–42. [DOI] [PubMed] [Google Scholar]
- 7.Rosendaal FR, Siscovick DS, Schwartz SM, et al. A common prothrombin variant (20210 G to A) increases the risk of myocardial infarction in young women. Blood 1997;90:1747–50. [PubMed] [Google Scholar]
- 8.Corral J, Gonzalez-Conejero R, Lozano ML, et al. The venous thrombosis risk factor 20210 A allele of the prothrombin gene is not a major risk factor for arterial thrombotic disease. Br J Haematol 1997;99:304–7. [DOI] [PubMed] [Google Scholar]
- 9.Watzke HH, Schuttrumpf J, Graf S, et al. Increased prevalence of a polymorphism in the gene coding for human prothrombin in patients with coronary artery disease. Thromb Res 1997;87:521–6. [DOI] [PubMed] [Google Scholar]
- 10.Arruda VR, Siquiera LH, Chiaparini LC, et al. Prevalence of the prothrombin gene variant 20210 G→A among patients with myocardial infarction. Cardiovasc Res 1998;37:42–5. [DOI] [PubMed] [Google Scholar]
- 11.Doggen CJ, Cats VM, Bertina RM, et al. Interaction of coagulation defects and cardiovascular risk factors: increased risk of myocardial infarction associated with factor V Leiden or prothrombin 20210A. Circulation 1998;97:1037–41. [DOI] [PubMed] [Google Scholar]
- 12.Eikelboom JW, Baker RI, Parsons R, et al. No association between the 20210 G/A prothrombin gene mutation and premature coronary artery disease. Thromb Haemost 1998;80:878–80. [PubMed] [Google Scholar]
- 13.Ridker PM, Hennekens CH, Miletich JP. G20210A mutation in prothrombin gene and risk of myocardial infarction, stroke, and venous thrombosis in a large cohort of US men. Circulation 1999;99:999–1004. [DOI] [PubMed] [Google Scholar]
- 14.Vargas M, Soto I, Pinto CR, et al. The prothrombin 20210A allele and the factor V Leiden are associated with venous thrombosis but not with early coronary artery disease. Blood Coagul Fibrinolysis 1999;10:39–41. [DOI] [PubMed] [Google Scholar]
- 15.Prohaska W, Schmidt M, Mannebach H, et al. The prevalence of the prothrombin 20210 G→A mutation is not increased in angiographically confirmed coronary artery disease. Thromb Haemost 1999;81:161–2. [PubMed] [Google Scholar]
- 16.Croft SA, Daly ME, Steeds RP, et al. The prothrombin 20210A allele and its association with myocardial infarction. Thromb Haemost 1999;81:861–4. [PubMed] [Google Scholar]
- 17.Inbal A, Freimark D, Modan B, et al. Synergistic effects of prothrombotic polymorphisms and atherogenic factors on the risk of myocardial infarction in young males. Blood 1999;93:2186–90. [PubMed] [Google Scholar]
- 18.Feng YJ, Draghi A, Linfert DR, et al. Polymorphism in the genes for coagulation factors II, V, and VII in patients with ischemic heart disease. Arch Pathol Lab Med 1999;123:1230–5. [DOI] [PubMed] [Google Scholar]
- 19.Ardissino D, Mannucci PM, Merlini PA, et al. Prothrombotic genetic risk factors in young survivors of myocardial infarction. Blood 1999;94:46–51. [PubMed] [Google Scholar]
- 20.Redondo M, Watzke HH, Stucki B, et al. Coagulation factors II, V, and X, prothrombin gene 20210 G→A transition, and factor V Leiden in coronary artery disease. High clotting activity is an independent risk factor for myocardial infarction. Arterioscl Thromb Vasc Biol 1999;19:1020–5. [DOI] [PubMed] [Google Scholar]
- 21.Burzotta F, Paciaroni K, De Stefano V, et al. Increased prevalence of the G20210A prothrombin gene variant in acute coronary syndromes without metabolic or acquired risk factors or with limited extent of disease. Eur Heart J 2002;23:26–30. [DOI] [PubMed] [Google Scholar]
- 22.Araújo F, Santos A, Araújo V, et al. Genetic risk factors in acute coronary disease. Haemostasis 1999;29:212–18. [DOI] [PubMed] [Google Scholar]
- 23.Coulet F, Godard V, Verdy E, et al. Lack of association of the prothrombin gene variant G20210A with myocardial infarction in Caucasian males. Thromb Haemost 2000;83:796–7. [PubMed] [Google Scholar]
- 24.Dilley A, Austin H, Hooper WC, et al. Prevalence of the prothrombin 20210 G-to-A variant in blacks, infants, patients with venous thrombosis, patients with myocardial infarction, and control subjects. J Lab Clin Med 1998;132:452–5. [DOI] [PubMed] [Google Scholar]
- 25.Pagano M, Gauvreau K. Principles of biostatistics. Duxbury: Wadsworth Inc, 1993.
- 26.Rosendaal FR, Doggen CJM, Zivelin A, et al. Geographic distribution of the 20210 G to A prothrombin variant. Thromb Haemost 1998;79:706–8. [PubMed] [Google Scholar]
- 27.Gardemann A, Arsic T, Katz N, et al. The factor II G20210A and factor V G1691A gene transitions and coronary heart disease. Thromb Haemost 1999;81:208–13. [PubMed] [Google Scholar]
- 28.Van de Water NS, French JK, Lund M, et al. Prevalence of factors V Leiden and prothrombin variant G20210A in patients age <45 years with no significant stenoses at angiography three to four weeks after myocardial infarction. J Am Coll Cardiol 2000;36:717–22. [DOI] [PubMed] [Google Scholar]
- 29.Burzotta F, Paciaroni K, Andreotti F, et al. G20210A prothrombin gene polymorphism and extent of coronary disease. Thromb Haemost 2000;84:142–3. [PubMed] [Google Scholar]
- 30.Wu AH, Tsongalis GJ. Correlation of polymorphisms to coagulation and biochemical risk factors for cardiovascular diseases. Am J Cardiol 2001;87:1361–6. [DOI] [PubMed] [Google Scholar]
- 31.Boekholdt AM, Bijsterveld NR, Moons AHM, et al. Genetic variation in coagulation and fibrinolytic proteins and their relation with acute myocardial infarction. A systematic review. Circulation 2001;104:3063–8. [DOI] [PubMed] [Google Scholar]
- 32.Di Castelnuovo A, De Gaetano G, Donati MB, et al. Platelet glycoprotein receptor IIIa polymorphism PlA1/A2 and coronary risk: a meta-analysis. Thromb Haemost 2001;85:626–33. [PubMed] [Google Scholar]
- 33.Choudhuy L, Marsh JD. Myocardial infarction in young patients. Am J Med 1999;107:254–61. [DOI] [PubMed] [Google Scholar]
- 34.Mikkelsson J, Karhunen PJ. Genetic variation in coagulation factors II, V, VII and fatal MI. Thromb Haemost 2002;87:349–50. [PubMed] [Google Scholar]
- 35.Maseri A. From syndromes to specific disease mechanisms. The search for the causes of myocardial infarction. Ital Heart J 2000;1:253–7. [PubMed] [Google Scholar]


