Myocardial infarction (MI) can be considered from several perspectives: clinical, electrocardiographic, biochemical, pathological, epidemiological, and imaging. The diagnosis of MI has psychological, social, and legal implications. MI is often used as a major end point in clinical trials.
WORLD HEALTH ORGANIZATION (WHO) DEFINITION
Historically, there has been tacit agreement as to the meaning of the term “myocardial infarction”. The World Health Organization definition, which has been widely used, requires the presence of two of the following three features: symptoms of myocardial ischaemia, elevation of cardiac marker (enzyme) concentrations in the blood, and a typical electrocardiographic pattern involving the development of Q waves or persistent T wave changes.w1
Using specific and highly sensitive immunoassays for myocardial proteins, such as cardiac troponins T and/or I, it is now possible to identify patients with small areas of myocardial necrosis. The emphasis on cardiac protein markers in the new American College of Cardiology/European Society of Cardiology (ACC/ESC) definition of MI, published in September 2000, has simplified the classification of MI.1 The new diagnostic criteria include a characteristic rise and fall in blood concentrations of cardiac troponins and/or creatine kinase (CK)-MB in the context of spontaneous ischaemic symptoms or coronary intervention (table 1).1
Table 1.
The new ACC/ESC definition of myocardial infarction (MI)1
Clinical features
|
Biochemistry
|
Electrocardiography
|
Pathology
|
Imaging
|
ACC, American College of Cardiology; CK, creatine kinase; ESC, European Society of Cardiology; MI, myocardial infarction
If it is accepted that any myocardial necrosis caused by ischaemia constitutes MI, many patients who were formerly diagnosed as having unstable angina pectoris will be now diagnosed as having had a small MI. For example, in a review of data from the Hennepin County Medical Centre (Minnesota, USA), the incidence of MI increased by 37% when the new definition of MI was applied.w2 However, the specificity of the new tests will reduce the number of false positive diagnoses of MI.
Under the WHO classification, which was expanded for the monitoring trends and determinants in cardiovascular disease (MONICA) epidemiological study,w3 patients could be classified as having definite or possible MI, prolonged angina pectoris, or ischaemic cardiac arrest. This system divided patients into six symptomatic categories, 27 electrocardiographic categories, and six enzymatic categories in 10 different combinations. The new ACC/ESC definition makes it possible to classify patients on the basis of seven clinical or pathological scenarios.
The limitations of the new definition of MI include the lack of a definition of cardiac arrest, and the lack of an MI classification for patients who present with characteristic symptoms of MI but die within 4–6 hours of symptom onset,w4 a window in which cardiac markers, the ECG, and histological findings (which take some hours to develop) may be non-diagnostic. Cardiac arrest and sudden cardiac death have different meanings for clinicians, epidemiologists, biochemists, and pathologists.w5 However, such events were also difficult to classify under the WHO-MONICA definition.w3 We believe that new definitions of MI are needed for patients suffering myocardial necrosis following coronary artery bypass grafting (CABG), and for those with silent MIs,w6 aborted MIs,w7 and threatened MIs; the latter group includes those with a thrombotic occlusion of the infarct artery without cardiac marker elevation.
CARDIAC MARKERS
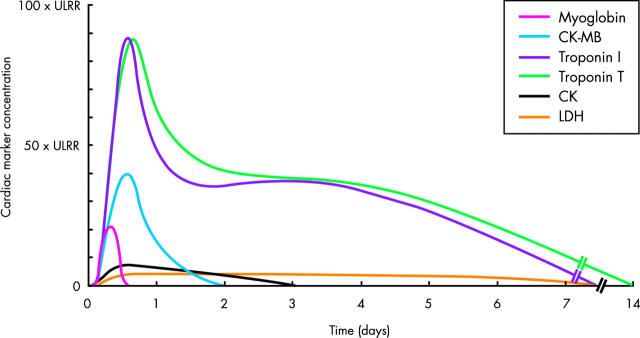
The traditional cardiac enzymes assayed for the detection of MI were the triad of lactate dehydrogenase (LDH), aspartate transaminase (also known as serum glutamate oxaloacetate transaminase), and CK. CK has isoenzymes of muscle (M) and brain (B) origin, and CK-MB may be distinguished from total CK both enzymatically and by immunoassay. The kinetic profiles of various cardiac markers in the blood are depicted in fig 1. Myocardial subfractions of the MM isoenzyme have been used for earlier detection of necrosis, largely for research applications.
Figure 1.
Kinetic profiles of cardiac markers following ST elevation myocardial infarction. These profiles are schematic and do not differentiate between patients with early reperfusion and those with persistent occlusion of the infarct related artery. When there is early reperfusion, cardiac marker concentrations rise more rapidly, peak earlier and at a higher value, and return to the reference range more rapidly.
To enable the early detection of MI after symptom onset, assays have been developed for proteins of smaller molecular mass, which appear more rapidly in the blood following the onset of necrosis. Myoglobin, with a molecular mass of 16 000 kD, is derived from the cytosol of both skeletal and cardiac muscle. It appears rapidly in the blood, and thus may have a specific role in the early detection of MI (table 2). Heart fatty acid binding protein is of cytosolic origin and has a similar small size and kinetic profile in the blood to that of myoglobin, but is more cardiospecific. However, neither myoglobin nor heart fatty acid binding protein have achieved widespread use as cardiac markers in clinical practice.
Table 2.
Properties of cardiac marker proteins
| Protein | Molecular mass (kD) | First detection* | Duration of detection | Sensitivity | Specificity |
| Fatty acid binding protein | 12 | 1.5–2 hours | 8–12 hours | +++ | ++ |
| Myoglobin | 16 | 1.5–2 hours | 8–12 hours | +++ | + |
| CK-MB | 83 | 2–3 hours | 1–2 days | +++ | +++ |
| Troponin I | 33 | 3–4 hours | 7–10 days | ++++ | ++++ |
| Troponin T | 38 | 3–4 hours | 7–14 days | ++++ | ++++ |
| CK | 96 | 4–6 hours | 2–3 days | ++ | ++ |
| Aspartate transaminase | ~103 | 6–10 hours | 3–5 days | ++ | + |
| LDH | 135 | 6–10 hours | 5–7 days | ++ | + |
*Hours after symptom onset. CK, creatine kinase; LDH, lactate dehydrogenase.
Over the past 15 years, immunoassays have been developed for the cardiac troponins T and I, which are components of the thin filaments of the sarcomere. Both are highly sensitive and highly specific and may be elevated when CK-MB concentrations are not (fig 2). Even minor elevations of troponin concentrations in the blood are thought to indicate myocyte necrosis; they are not thought to be caused by leakage of proteins due to reversible permeability of the myocyte cell membrane. The current assays for troponins T and I reliably detect cardiac (as distinct from skeletal muscle) forms of these proteins. While several manufacturers have produced assays for troponin I, patent protection has meant that only Roche Diagnostics (Basel, Switzerland; formerly Boehringer Mannheim of Mannheim, Germany) has produced a troponin T assay. There have been three generations of this laboratory assay, and the current laboratory troponin T assay has a high degree of correlation with the point-of-care (bedside) assay. Point-of-care cardiac marker testing provides results within minutes, and should be used when the delay in obtaining laboratory results is likely to exceed 60 minutes.2w8
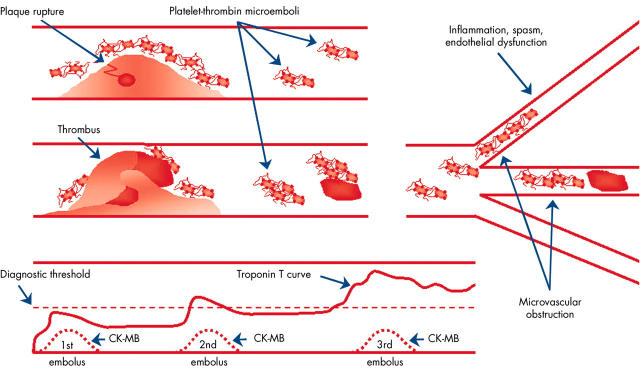
Figure 2.
Microvascular obstruction after plaque rupture, and temporal release of CK-MB and troponins during repetitive episodes of ischaemia causing myocardial necrosis in the setting of an acute coronary syndrome. Unlike the release and clearance of CK-MB 48–72 hours after each episode (indicated as 1st, 2nd and 3rd), troponin T concentrations increase cumulatively, partly due to slower clearance. Adapted with permission from: Goldmann BU, et al. Implications of troponin testing in clinical medicine. Curr Control Trials Cardiovasc Med 2001;2:75–84.
Using the current generation troponin T assay, the discrimination value for the detection of MI is 0.03 μl, which is higher than the 99th centile of the reference group values. At 0.03 μg/l the coefficient of variation is ⩽ 10%, which meets the level of precision specified in the ACC/ESC definition.1 If the 10% coefficient of variation for a particular troponin I assay occurs at a discrimination value of 0.1 μg/l, there is only one chance in 20 that a value will be reported outside the range of 0.09–0.11 μg/l. Different manufacturers have used different antibodies raised against different epitopes on cardiac troponin I, and there is no standardisation between the different troponin I assays,w8, w9 which complicates their use.w10 Troponin I values may also be affected by heterophil antibodies. At the discrimination values for troponin I, the assays do not consistently achieve coefficients of variation of < 10%, and these concentrations are not higher than the 99th centile of the reference control group.w11, w12 Thus at present, no troponin I assay meets the ACC/ESC criteria for diagnosis of MI.1
Entry criteria for clinical trials evaluating treatments for acute coronary syndromes are increasingly based on troponin values. Use of imprecise assays could lead to diagnostic inconsistencies between different centres and/or regions, potentially confounding trial results. Thus the lack of standardisation of discrimination values for troponin I could compromise the ability to apply trial results to the general population.
CARDIAC MARKERS AND CLINICAL OUTCOMES
Acute coronary syndromes
Compared with other protein markers of myocardial necrosis, troponins have greater sensitivity and specificity for the diagnosis of MI in the setting of acute myocardial ischaemia.w13–15 In both short and long term follow up studies, the magnitude of troponin elevations has correlated consistently with the risk of death and the composite risk of death or non-fatal MI, irrespective of whether the patients had ST elevation or non-ST elevation acute coronary syndromes.3 w16–19 In the global use of strategies to open occluded coronary arteries in acute coronary syndromes (GUSTO)-IIa troponin T substudy, the baseline troponin T value correlated with the risk of mortality at 30 days, and was the second most powerful predictor after electrocardiographic changes.w20 The thrombolysis in myocardial infarction (TIMI) group reported similar findings using the Dade-Behring troponin I assay (Deerfield, Illinois, USA), with the risk ratio for mortality at 42 days increasing in a linear manner with increasing troponin values (relative risk 7.8, 95% confidence interval (CI) 2.6 to 23.0, for troponin I values of < 0.4 v > 9 μg/l).w19 Troponin values have been shown to be a more powerful prognostic indicator than CK-MB,3w20 and serial sampling (as opposed to single sampling) enhances the likelihood of identifying myocyte necrosis among patients presenting at different stages of the infarction process.w21
Acceptance of troponin testing for the diagnosis of MI
A recent survey in Scotland found that only 70% of cardiologists had access to troponin assays, and sometimes only by special request.w22 Furthermore, only one third of cardiologists made a diagnosis of MI in patients presenting with typical chest pain and elevated troponin concentrations in the absence of typical electrocardiographic changes or elevated CK or CK-MB values.w22 In another survey conducted in 25 European and Mediterranean basin countries4 nine months after the publication of the new ACC/ESC definition of MI, > 20% of patients receiving a diagnosis of MI did not have elevated troponin or CK values, while a similar percentage received a diagnosis of unstable angina despite having elevated CK or troponin values.4 Experience from clinical trials has shown that ~2% of patients receiving a diagnosis of MI die before their cardiac marker concentrations reach the threshold for detection,w23 while another 1–3% have their MI successfully “aborted” by early treatment.w27 The timing of assays was not documented in this survey; however, the likely explanation in most cases is that the survey was perhaps conducted too soon after publication of the new ACC/ESC definition for the majority of clinicians to have incorporated the new diagnostic criteria into their clinical practice, and it is likely that many patients were given a diagnosis of MI because they met the WHO-MONICA criteria of ischaemic symptoms and electrocardiographic changes. However, it is perturbing that at least one fifth of all patients with MI and unstable angina were “misclassified” in both directions according to the new ACC/ESC definition.
Abbreviations and trial acronyms.
ACC: American College of Cardiology
ARTS: Arterial Revascularization Therapies Study
CI: confidence interval
CK: creatine kinase
ESC: European Society of Cardiology
GUSTO: Global Use of StraTegies to Open occluded coronary arteries in acute coronary syndromes
HERO: Hirulog and Early Reperfusion or Occlusion
LDH: lactate dehydrogenase
MI: myocardial infarction
MONICA: MONItoring trends and determinants in CArdiovascular disease
PCI: percutaneous coronary intervention
RITA: Randomised Intervention Trial of unstable Angina
TIMI: Thrombolysis In Myocardial Infarction
ULRR: upper limit of the reference range
WHO: World Health Organization
Troponins are very sensitive markers of myocyte necrosis, and troponin elevations can occur in settings other than spontaneous ischaemia or percutaneous coronary intervention (PCI) (table 3). Apart from acute coronary syndromes, the most frequent causes of elevated troponin concentrations are tachycardia (with or without hypotension), pulmonary emboli with right ventricular infarction,w24 and cardiac failure with myocardial necrosis caused by neurohumoral changes and elevated left ventricular end diastolic pressure. Other causes of elevated troponin values include cardiac surgery, myocarditis, and renal failure, in which the cause of myocyte necrosis is yet to be elucidated.5
Table 3.
Causes of elevated troponin values in clinical settings other than acute coronary syndromes or percutaneous coronary intervention
Ischaemic causes other than plaque fissuring or rupture
|
Cardiac surgery
|
Miscellaneous
|
Myopericarditis
|
Infiltrative diseases of the myocardium
|
Traumatic
|
Diagnosis of reinfarction
The use of troponins as the primary markers of myocardial necrosis presents a significant challenge for the detection of reinfarction, because they have different kinetic profiles according to whether the culprit artery is occluded or patent, and they have a long half life of up to 14 days in the blood (fig 1). Thus the interpretation of changes in troponin values during this time is problematic. Algorithms have been developed for the diagnosis of reinfarction following both ST elevation and non ST-elevation acute coronary syndromes,w23, w25, w26 predominantly using CK-MB in instances of “spontaneous” recurrent ischaemia and following PCI or surgical revascularisation. Table 4 lists the criteria used to diagnose reinfarction in the hirulog and early reperfusion or occlusion (HERO)-2 trial, which was the first multicentre mega-trial conducted in patients receiving fibrinolytic treatment to have all cases of reinfarction adjudicated by a clinical end points committee.w23 There has been a recent report6 describing the use of troponins to diagnose reinfarction, and these findings require validation.
Table 4.
Definition of reinfarction in the HERO-2 trialw23
| Clinical scenario | Criteria |
| Recurrent symptoms occurring within 18 hours | Chest pain lasting ⩾30 minutes |
| and ⩾2 mm of ST elevation | |
| Recurrent symptoms occurring after lasting >18 hours | CK rise to >2 × ULRR and >50% above previous baseline value |
| or CK-MB value >ULRR and >50% above previous baseline value | |
| or new left bundle branch block or new Q waves | |
| Following PCI | CK rise to >3 × ULRR |
| or CK-MB value >3 × ULRR | |
| or new left bundle branch block or new Q waves | |
| Following CABG | CK rise to >5 × ULRR |
| or CK-MB value >5 × ULRR | |
| or new left bundle branch block or new Q waves |
CABG, coronary artery bypass grafting; CK, creatine kinase; PCI, percutaneous coronary intervention; ULRR, upper limit of the reference range.
Cardiac marker elevations occurring in the context of percutaneous coronary intervention
Myocyte necrosis indicated by elevation of cardiac marker concentrations occurs frequently in the absence of clearly definable clinical events after an otherwise successful PCI, and is associated with adverse clinical outcomes, including death.7,8w27–32 Detectable rises in CK-MB values occur following 5–30% of PCI procedures, and have been associated with adverse outcomes (death, MI, or repeat urgent target vessel revascularisation) in both short and long term follow up studies.7 w27, w29–31, w33 The relation between periprocedural CK-MB elevations and late mortality is approximately linear, and myocardial necrosis has similar prognostic significance whether it is caused by a “spontaneous” ischaemic event or by PCI.9
Periprocedural rises in troponin concentrations occur in up to 40% of cases,w34–48 and appear to have prognostic significance similar to that of elevated CK-MB values. In one study, 26% of patients with normal troponin values before PCI were found to have concentrations above the upper limit of the reference range (ULRR) after PCIw35; these patients had a 90 day mortality rate of 5.2% compared with 0% in those without elevated troponin I values after PCI (hazard ratio 4.3, 95% CI 1.4 to 13.5, for 90 day death or MI). Another recent study of 1872 patients found that 32% developed elevated troponin I concentrations after PCI, and that elevated troponin I values (odds ratio 1.7), the presence of diabetes mellitus (odds ratio 3.0), and older age (odds ratio 1.1) were all independent predictors of one year mortality.w34
Cardiac marker elevations following PCI may be caused by occlusion of small branch arteries or intimal disruption, but the usual pathophysiology is thought to be embolisation of platelet aggregates (fig 2) or plaque constituents. Utilisation of distal protection devices during PCI has demonstrated the presence of this embolic debris.10,11w25, w49–53 Glycoprotein IIb/IIIa receptor antagonists have been shown to reduce the incidence of periprocedural MI, and the reduction in long term mortality seen with abciximab treatment supports the clinical importance of platelet emboli.12 There is controversy as to whether the adverse long term prognosis associated with periprocedural MI is due to small areas of myocyte necrosis, or whether periprocedural cardiac marker elevations reflect a large atherosclerotic burden in the coronary arteries and elsewhere.w29 The majority of deaths occurring after periprocedural MI have been reported as being sudden, which suggests an arrhythmic mechanism possibly related to multiple small areas of myocardial necrosis.8
The reported incidence of “spontaneous” MI in acute coronary syndrome trials where PCI was not mandated has been higher than the incidence of periprocedural MI observed in trials of PCI. The unadjusted odds ratios for increasing cardiac marker values (relative to normal) in these two scenarios were similar (approximately twofold for elevations of 1–5 times the ULRR, and fourfold for elevations of 5–10 times the ULRR).9 These data support the hypothesis that, regardless of aetiology, any myocyte necrosis has prognostic implications,9 but that other factors known to be prognostically significant also need to be taken into account.
Cardiac marker elevations occurring in the context of coronary artery bypass grafting
Almost all patients undergoing CABG have some elevation of cardiac markers, and 20–40% of patients have pronounced elevations.w54 Earlier studies, which used electrocardiographic and CK criteria for the diagnosis of MI following CABG, reported an association between perioperative MI, heart failure, and mortality.13,14w55, w56 Of 9777 patients enrolled in the coronary artery surgery study (CASS) registryw57 between 1974 and 1979, 5.7% had definite or probable perioperative MI. Hospital survivors with perioperative infarction were found to have a significantly lower five year survival rate than those without perioperative MI (40% v 73%).w58
In one study, the best combination of specificity (85%) and sensitivity (39%) for adverse six month outcomes associated with post-CABG CK-MB elevations was shown to be at a discrimination value of five times the ULRR.w27 In another study of 390 patients undergoing CABG after acute coronary syndromes,w54 CK-MB elevation to < 5 times the ULRR was not associated with significant increases in adverse events occurring in hospital, within 30 days or within six months; however, CK-MB elevation to ⩾ 5 times the ULRR was associated with a twofold increase in six month mortality (p = 0.001), and elevation to ⩾ 10 times the ULRR was associated with an almost fourfold increase in mortality at both 30 days and six months.
In the guard during ishemia against necrosis (GUARDIAN) study,w59 in which 2918 patients underwent CABG and postoperative MI was a major end point, increasing values of CK-MB above the ULRR correlated with six month mortality after CABG. The odds ratios for six month mortality were 1.74 (95% CI 1.05 to 2.86) in patients with CK-MB values of 5–10 times the ULRR, and 2.38 (95% CI 1.32 to 4.28) in those with CK-MB values of 10–20 times the ULRR.w59
CK-MB elevations in the range of 3–5 times the ULRR have been considered to have different prognostic implications, depending on whether they occur in the context of CABG or PCI.w27 However, in the arterial revascularization therapies study (ARTS),15 where 496 patients underwent CABG, the one year mortality rates were 1.1% in patients with normal CK-MB values postoperatively, 0.5% in those with values of > 1–3 times the ULRR, 5.4% in those with values of 3–5 times the ULRR, and 10.5% in those with values of > 5 times the ULRR (p < 0.001).15 There was an association between increasing CK-MB elevations and mortality that persisted after adjustment for baseline characteristics such as age and left ventricular function, and was similar to that observed with spontaneous or PCI related MI.15
The pattern of troponin release is similar after CABG and after valve surgery,w60 although perioperative troponin concentrations are substantially lower in patients undergoing off-pump or minimally invasive surgery.w61–66 There are few published data on the association between troponin elevations following CABG and clinical outcomes. In a small study of 45 patients undergoing elective CABG for two vessel coronary artery disease, higher troponin T values were associated with delayed recovery of left ventricular function.w67 In another study of 540 consecutive patients undergoing elective CABG at a single centre, the median perioperative troponin I values (using the Dade-Behring assay) were 0.91 μg/l in 21 patients who subsequently died or had an in-hospital MI v 0.37 μg/l in those who did not have these events.w68 In multivariate analysis, the only factors that predicted the in-hospital outcome were the cross-clamp time and the troponin I value at discontinuation of bypass.
The ACC/ESC definition does not include specific criteria for the diagnosis of MI occurring post-CABG.w68–77 Not all causes of myocyte necrosis following CABG involve ischaemia caused by plaque fissuring or rupture (table 3), and cardiac marker elevation after CABG can be caused by traumatic atrial cannulation, ventricular venting, manipulation of the heart, inadequate cardioplegia, or an ischaemia related event such as conduit or native vessel occlusion due to thrombosis or spasm.
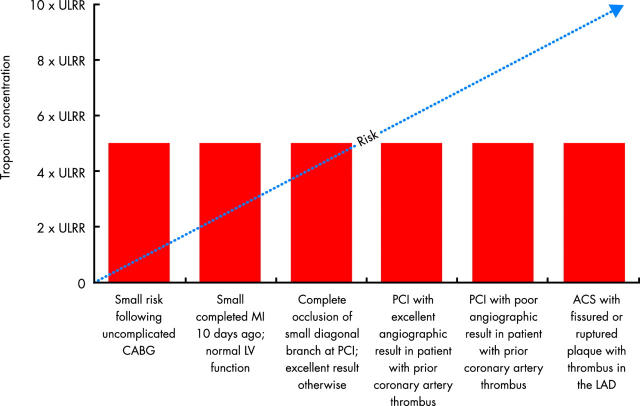
Cardiac marker elevations post-CABG that do not reach the commonly used discrimination value for MI (five times the ULRR) may still have prognostic implications, though these may be less important than in other clinical scenarios (fig 3). Based on the ARTS data, the CK-MB threshold associated with an increase in late mortality after CABG may be three rather than five times the ULRR, although this will need to be validated in future studies. A troponin based definition of MI occurring in the context of CABG is yet to be established.
Figure 3.
The prognostic significance of troponin elevations varies in different clinical scenarios. ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; LAD, left anterior descending coronary artery; LV, left ventricular; PCI, percutaneous coronary intervention; ULRR, upper limit of reference range. Modified from White HD. Things ain’t what they used to be: impact of a new definition of myocardial infarction [editorial]. Am Heart J 2002;144:933–7, with permission.
Definitions of MI in clinical trials
In recent clinical trials of antithrombotic treatment in acute coronary syndromes, the cardiac marker definition of MI has relied primarily on elevations of CK or CK-MB.w25, w78–81 These definitions have varied between trials and according to the clinical scenario (for example, 1–2 times the ULRR for spontaneous MI, > 3 times the ULRR for PCI related MI, and > 5 times the ULRR for CABG related MI), and may also vary depending on whether troponin values are included in the definition.w82
Clinical implications of the new definition of MI: key points.
The new definition of myocardial infarction (MI), based on troponin values, will increase the number of patients with non-ST elevation acute coronary syndromes receiving a diagnosis of MI by about 40%
The new definition of MI, based on troponin values, has not yet been universally adopted by cardiologists
The available troponin I assays require further refinement to achieve a coefficient of variation of < 10% at the 99th centile of the reference control group, and standardisation of troponin I assays should be pursued vigorously
There are similar associations between mortality and cardiac marker values following MI in the settings of acute myocardial ischaemia, post-PCI, and post-CABG
- New definitions are needed for:
- –MI post-CABG based on postoperative CK-MB and troponin values
- –threatened infarction
- –aborted MI
- –silent MI
- –sudden ischaemic cardiac death
Unlike other studies comparing early invasive and conservative treatment strategies in patients with non-ST elevation acute coronary syndromes,w83 the recent randomised intervention trial of unstable angina (RITA-3) study16 used the same criteria (elevation of cardiac marker concentrations to > 2 times the ULRR) to define “spontaneous” MI and PCI related MI. If the RITA-3 data are re-analysed using the new ACC/ESC definition of MI (in which any elevation in cardiac marker values above the ULRR is defined as an MI), the number of MIs occurring in the invasive versus conservative treatment groups increases from 45 v 56 MIs (p = NS) to 84 v 129 MIs (risk ratio 0.67, 95% CI 0.51 to 0.86, p = 0.002). These data emphasise just how much the interpretation of trial results depends upon the definition of MI being used, especially when intervention rates differ within and/or between studies.
Prevention of reinfarction should be considered an important end point in assessing the potential benefits of new cardiovascular therapies, as reinfarction is associated with decreased late survival.17 Reduction in infarct size should also be a therapeutic goal and, given the association between the magnitude of cardiac marker elevations and mortality rates, it may be appropriate to consider cardiac marker values as continuous variables rather than as discrete variables in data analysis.w25
SOCIETAL IMPLICATIONS OF THE NEW DEFINITION OF MI
In addition to changing the prognosis of patients with MI (by lowering the threshold for diagnosis), the new definition will have various implications for society. Its effect on global healthcare expenditure is as yet unknown. Hospital costs are likely to rise, as patients diagnosed with MI will be more likely to undergo investigations such as echocardiography and/or angiography and PCI. However, more accurate diagnosis is also likely to lead to more appropriate usage of evidence based treatment and consequently better patient outcomes, which may reduce the overall costs of coronary heart disease to society.
The lower diagnostic threshold for MI will also have a considerable impact upon patients and their families. Receiving a diagnosis of MI may prompt more patients to change their lifestyles, but may also affect patients’ ability to retain or regain employment, with consequences for productivity and health insurance. Community education programmes will be needed to inform the public that the prognosis of patients who have had a small troponin rise is usually excellent, provided that appropriate remedial measures are taken (for example, lifestyle modification including exercise; pharmacological treatments such as aspirin, β blockers, statins, and angiotensin converting enzyme inhibitors; and PCI or CABG in selected patients).
Guidelines for resumption of driving after an MI will need to be modified. The latest New Zealand driving guidelines have taken into account the new definition, and allow patients to resume driving two days after an uncomplicated PCI or one week after presentation with a non-ST elevation acute coronary syndrome involving a small troponin rise. Guidelines for resumption of flying will also need to be revised. It is important that the definition of MI is standardised both nationally and internationally. We believe that the new definition of MI is a major advance, and will lead to improved patient management and outcomes.
Supplementary Material
REFERENCES
- 1.The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined - a consensus document of the joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:150–13 (Also published in J Am Coll Cardiol 2000;36: 959–69. ▸ This consensus document redefines myocardial infarction and discusses some of the issues raised by the new definition. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E, Antman EM, Beasley JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). Circulation 2000;102:1193–209. [DOI] [PubMed] [Google Scholar]
- 3.Hamm CW, Ravkilde J, Gerhardt W, et al. The prognostic value of serum troponin T in unstable angina. N Engl J Med 1992;327:146–50. ▸ This is the first published report examining the prognostic value of troponin T values in patients with unstable angina. [DOI] [PubMed] [Google Scholar]
- 4.Hasdai D, Behar S, Boyko V, et al. Cardiac biomarkers and acute coronary syndromes – the Euro-Heart Survey of Acute Coronary Syndromes experience. Eur Heart J 2003;24:1189–94. ▸ This article surveys the use of troponin testing in the Mediterranean region approximately nine months after publication of the new diagnostic criteria, and notes that at least 20% of patients diagnosed as having had a myocardial infarction did not have confirmation of elevated cardiac marker values. [DOI] [PubMed] [Google Scholar]
- 5.Aviles RJ, Askari AT, Lindahl B, et al. Troponin T levels in patients with acute coronary syndromes, with or without renal dysfunction. N Engl J Med 2002;346:2047–52. [DOI] [PubMed] [Google Scholar]
- 6.Newby LK, Goldmann BU, Ohman EM. Troponin: an important prognostic marker and risk-stratification tool in non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol 2003;41:S31–6. [DOI] [PubMed] [Google Scholar]
- 7.Abdelmeguid AE, Topol EJ, Whitlow PL, et al. Significance of mild transient release of creatine kinase-MB fraction after percutaneous coronary interventions. Circulation 1996;94:1528–36. [DOI] [PubMed] [Google Scholar]
- 8.Brener SJ, Ellis SG, Schneider J, et al. Frequency and long-term impact of myonecrosis after coronary stenting. Eur Heart J 2002;23:869–76. ▸ This review discusses the pathophysiological mechanism, management implications, and prognostic importance of cardiac marker elevations occurring after intracoronary stent deployment. [DOI] [PubMed] [Google Scholar]
- 9.Akkerhuis KM, Alexander JH, Tardiff BE, et al. Minor myocardial damage and prognosis: are spontaneous and percutaneous coronary intervention-related events different? Circulation 2002;105:554–6. ▸ This paper concludes that small elevations in cardiac markers (implying the occurrence of minor myocardial necrosis) have similar prognostic implications irrespective of whether they occur spontaneously or in the context of percutaneous coronary intervention. [DOI] [PubMed] [Google Scholar]
- 10.Grube E, Gerckens U, Yeung AC, et al. Prevention of distal embolization during coronary angioplasty in saphenous vein grafts and native vessels using porous filter protection. Circulation 2001;104:2436–41. [DOI] [PubMed] [Google Scholar]
- 11.Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease. Circulation 2000;101:570–80. ▸ This review discusses the importance of distal embolisation and its prevention in various clinical manifestations of atherosclerotic vascular disease. [DOI] [PubMed] [Google Scholar]
- 12.Chew DP, Roffi M, Topol EJ. Intravenous glycoprotein IIb/IIIa inhibition in non-ST segment elevation acute coronary syndromes. Prog Cardiovasc Dis 2001;44:195–206. [DOI] [PubMed] [Google Scholar]
- 13.Brewer DL, Bilbro RH, Bartel AG. Myocardial infarction as a complication of coronary bypass surgery. Circulation 1973;47:58–64. [DOI] [PubMed] [Google Scholar]
- 14.Chaitman BR, Alderman EL, Sheffield LT, et al. Use of survival analysis to determine the clinical significance of new Q wave after coronary bypass surgery. Circulation 1983;67:302–9. [DOI] [PubMed] [Google Scholar]
- 15.Costa MA, Carere RG, Lichtenstein SV, et al. Incidence, predictors, and significance of abnormal cardiac enzyme rise in patients treated with bypass surgery in the arterial revascularization therapies study (ARTS). Circulation 2001;104:2689–93. ▸ This is the first report describing the relative prognostic significance of the extent of CK-MB elevations in patients undergoing coronary artery bypass grafting. [DOI] [PubMed] [Google Scholar]
- 16.Fox K, Poole-Wilson P, Henderson R, et al. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Lancet 2002;360:743–51. ▸ This paper emphasises the importance of the definitions used when interpreting trial results. Data analysis based on older cardiac marker criteria showed no reduction in infarction with invasive treatment, whereas the reduction was significant when the data were re-analysed according to the new ACC/ESC definition. [DOI] [PubMed] [Google Scholar]
- 17.Hudson MP, Granger CB, Topol EJ, et al. Early reinfarction after fibrinolysis: experience from the global utilization of streptokinase and tissue plasminogen activator (alteplase) for occluded coronary arteries (GUSTO I) and global use of strategies to open occluded coronary arteries (GUSTO III) trials. Circulation 2001;104:1229–35. ▸ This paper describes the adverse prognostic influence of early reinfarction on one year survival in the GUSTO-I and GUSTO-III mega-trials. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.