Abstract
Objective: To determine the left ventricular (LV) activation pattern in patients with chronic heart failure and left bundle branch block (LBBB) on ECG.
Design: Prospective study.
Setting: Tertiary cardiology referral centre in Hong Kong.
Patients: Seven patients with LV ejection fraction < 35% and typical LBBB on ECG with QRS duration ⩾ 130 ms were recruited. Five of them had non-ischaemic dilated cardiomyopathy.
Methods: Non-contact mapping was used to investigate the LV global activation sequences. Tissue Doppler imaging was performed with the LV mapping and correlated with the activation sequences.
Results: Three patients had preserved left bundle activation despite LBBB on ECG. Conduction block was detected in four patients during LV activation and the other three had homogeneous depolarisation propagation within the left ventricle. The latest segment of activation was located in either the lateral or the posterior region. Tissue Doppler imaging correlated well with non-contact mapping to locate the conduction block and the latest segment of activation.
Conclusions: LV endocardial activation sequences in patients with chronic heart failure and LBBB are variable. This may have implications for patient selection for treatment with cardiac resynchronisation.
Keywords: heart failure, bundle branch block, electrophysiology
Cardiac resynchronisation improved left ventricular (LV) function in patients with chronic heart failure and left bundle branch block (LBBB).1 Mechanical asynchrony, as shown by tissue Doppler imaging (TDI), exists in these patients and correction by cardiac resynchronisation may be the underlying mechanism for clinical improvement.2 However, some patients failed to respond to cardiac resynchronisation and the reason remains unclear. Different LV pacing sites produced variable improvement of LV systolic performance in acute haemodynamic studies. Pre-excitation of the delayed LV region by pacing may improve ventricular asynchrony.3 The location of the delayed LV segment may be related to the site of conduction delay within the left ventricle. Thus, LV electrical activation sequences may be vital for selecting the optimal LV pacing site and useful for predicting the response to cardiac resynchronisation. We examined the application of non-contact mapping and TDI to delineate LV activation sequences in patients with low LV ejection fraction and LBBB. Our hypothesis was that LV activation sequences are variable in these patients and this may account for the different responses to cardiac resynchronisation.
METHODS
Seven patients with New York Heart Association class III with ejection fraction < 35% and LBBB morphology with QRS duration ⩾ 130 ms were studied. The mean (SD) age was 66.1 (7.3) years, QRS duration was 152 (19) ms, and ejection fraction was 23 (7)%. Five patients had non-ischaemic heart failure. All patients were in sinus rhythm and received optimal medical treatment. Written informed consent was obtained from all patients. LV activation was mapped and TDI assessed simultaneously.
The LV activation pattern was delineated by non-contact mapping as previously described.4 The left ventricle was divided into basal, mid, and apical sections in a longitudinal axis. Each section was divided further into anterior, posterior, lateral, and septal regions, giving 12 LV segments for analysis. The earliest and latest segments of activation together with the propagation pattern were determined by tracking back in time in the isopotential colour maps. Left bundle activation was evident by the earliest site of activation detected after the His signal in the basal septal region of the left ventricle.
TDI was assessed as previously described.2 Myocardial pulse-Doppler velocity signals were reconstituted from the TDI colour images that provided regional myocardial velocity curves. A total of 12 segments, similar to those of non-contact mapping, were analysed. The time to peak myocardial sustained systolic velocity (TS) was measured in each segment by using the onset of the QRS complex as the reference point. The earliest and latest LV segments together with the propagation pattern detected by both methods were compared.
RESULTS
Preserved left bundle branch activation was detected in three patients. The earliest LV activation was located in the apical anterior and basal septal segments in the other four patients. The earliest LV activation segment by non-contact mapping corresponded well to the earliest TS segment by TDI (fig 1).
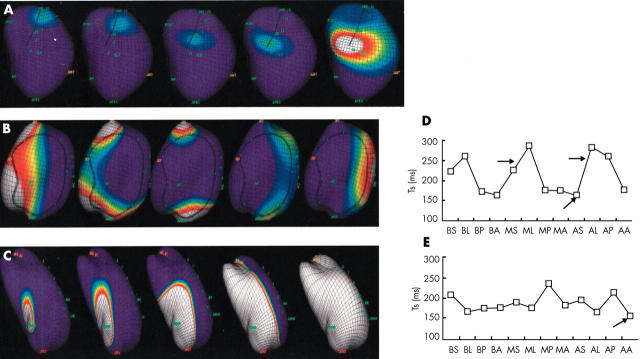
Figure 1.
(A–C) Isopotential maps showing the left ventricular (LV) activation sequences. (A) From left to right: left bundle branch activation. (B) Activation sequences across the LV anterior wall. The depolarisation wavefront splits when approaching the conduction block area (circled by the dark line). The two wavefronts merged in the lateral wall. (C) The endocardial activation originated from the apical anterior area and propagated across the LV without acute change of direction. (D and E) Corresponding sequences of LV systolic movement in various segments shown by tissue Doppler imaging. (E) The earliest peak systolic movement was located at the apical anterior segment (arrow). There was “homogeneous” propagation across the left ventricle as reflected by relatively minor difference in TS between the LV segments. (D) The earliest peak systolic movement was located at the apical septal area corresponding to left bundle activation (lower arrow). There was a major difference in TS particularly between septal and lateral LV regions (upper arrows), which was related to the anterolateral conduction block detected by isopotential maps. AA, apical anterior; AL, apical lateral; AP, apical posterior segment of LV wall; AS, apical septal; BA, basal anterior; BL, basal lateral; BP, basal posterior; BS, basal septal; MA, midanterior; ML, mid lateral; MP, mid posterior; MS, mid septal; Ts, time from the onset of QRS complex to peak systolic velocity in each LV segment by tissue Doppler imaging.
Two distinct patterns of LV propagation were observed. “Homogeneous” propagation was observed in three patients (type I). There was no acute change in propagation direction during the whole LV depolarisation sequences. Conduction block was observed in the other four patients (type II). There was an acute change in propagation direction and the wavefront split and turned around a region with relatively low voltage in the isopotential map (fig 1).
Consistent findings between non-contact mapping and TDI assessment were also observed in detecting the latest segment of activation and systolic movement (fig 1). The latest segments were located in the basal and midlateral area in three patients and in the mid and apical posterior area in four patients.
DISCUSSION
The current study found that the LV depolarisation sequences in patients with chronic heart failure and LBBB were variable. Left bundle activation was preserved in three non-ischaemic patients despite LBBB. The LV conduction delay may have resulted from either an area of conduction block or slow but homogeneous myocardial propagation. The nature of the conduction block needs to be clarified by future studies. Whether the block was caused by scarred tissue or was functional cannot be determined from the present study. In those with preserved left bundle activation, prolonged LV activation time was likely caused by slow myocardial electrical propagation but not conduction block in one patient. Both types of conduction delay were present in ischaemic and non-ischaemic heart failure. Moreover, the location of the type II block varied between patients. Anterolateral conduction block was observed in two patients and septal block in two patients. For the latest segment of activation, both posterior and lateral locations were observed in this cohort. These findings also suggested that ECG with LBBB could not predict the LV activation sequences. Whether these finding account for the different response to cardiac resynchronisation requires further investigation.
Detailed information about mechanical asynchrony in patients with heart failure and LBBB has been reported previously.2 However, information about the LV depolarisation sequences of these patients is lacking. The significance of preserved left bundle activation in these patients is unclear. In two patients with true left bundle blockade, the earliest activation was in apical anterior area rather than directly across the interventricular septum as generally believed. This anomaly may result from subendocardial conduction or other unknown mechanism that could not be detected by non-contact mapping.
The latest LV activation segments were located in either the lateral or posterior regions in this study. This finding is consistent with a study in which TDI was used to assess improved synchronicity after biventricular pacing, which found that systolic motion was latest in the lateral segments.2 However, in four patients, the latest segments were located in the posterior region. If sustained clinical improvement is related to pre-excitation of the latest segment, lateral placement of the lead may not always be optimal.
In conclusion, the LV endocardial activation sequences in patients with chronic heart failure and LBBB are variable. Left bundle activation was preserved in some patients with LBBB. TDI correlated well with non-contact mapping for locating the latest segment of activation in these patients and may guide the selection of optimal sites for biventricular pacing.
Abbreviations
LBBB, left bundle branch block
LV, left ventricular, TDI, tissue Doppler imaging
TS, time to peak myocardial sustained systolic velocity
REFERENCES
- 1.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–53. [DOI] [PubMed] [Google Scholar]
- 2.Yu CM, Chau E, Sanderson JE, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002;105:438–45. [DOI] [PubMed] [Google Scholar]
- 3.Butter C, Auricchio A, Stellbrink C, et al. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation 2001;104:3026–9. [DOI] [PubMed] [Google Scholar]
- 4.Schilling RJ, Peters NS, Davies DW. Mapping and ablation of ventricular tachycardia with the aid of a non-contact mapping system. Heart 1999;81:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]