Abstract
The fruit fly Drosophila melanogaster was used to examine the mode of action of the novel insecticide and acaricide nodulisporic acid. Flies resistant to nodulisporic acid were selected by stepwise increasing the dose of drug in the culture media. The resistant strain, glc1, is at least 20-fold resistant to nodulisporic acid and 3-fold cross-resistant to the parasiticide ivermectin, and exhibited decreased brood size, decreased locomotion, and bang sensitivity. Binding assays using glc1 head membranes showed a marked decrease in the affinity for nodulisporic acid and ivermectin. A combination of genetics and sequencing identified a proline to serine mutation (P299S) in the gene coding for the glutamate-gated chloride channel subunit DmGluClα. To examine the effect of this mutation on the biophysical properties of DmGluClα channels, it was introduced into a recombinant DmGluClα, and RNA encoding wild-type and mutant subunits was injected into Xenopus oocytes. Nodulisporic acid directly activated wild-type and mutant DmGluClα channels. However, mutant channels were ≈10-fold less sensitive to activation by nodulisporic acid, as well as ivermectin and the endogenous ligand glutamate, providing direct evidence that nodulisporic acid and ivermectin act on DmGluClα channels.
Because of the development of resistance and the search for less toxic drugs, there is a continuous need for novel antiparasitic compounds. A blowfly (Lucilia seracata) killing assay identified the natural product nodulisporic acid as a potent insecticide (1). Isolated from Nodulisporium sp., an endophytic fungus, nodulisporic acid is an indole terpene that effectively kills not only blowflies, but also mosquitoes (Aedes aegypti) and fruit flies (Drosophila melanogaster) (1, 2). In the blowfly and mosquito assays, nodulisporic acid was more potent than paraherquamide, malathion, and DDT, but less potent than ivermectin (1, 2).
Although the basis of the drug's insecticidal activity was not known, recent studies indicate that nodulisporic acid acts by a mechanism similar to that of ivermectin (3). In grasshopper metathoracic ganglia, nodulisporic acid, like ivermectin, activates a chloride conductance. No additional current is seen when nodulisporic acid is added after application of ivermectin. Furthermore, binding of radiolabeled nodulisporic acid to Drosophila head membranes is inhibited completely in the presence of saturating concentrations of ivermectin. Ivermectin, a macrocyclic lactone, is known to activate recombinant glutamate-gated chloride (GluCl) channels (4, 5). At least in Caenorhabditis elegans, the effect on GluCl channels underlies the anthelmintic activity of ivermectin and its homologues. A C. elegans strain, avr-14;avr-15;glc-1, carrying simultaneous mutations of the three GluCl channel α-type subunits α1, α2, and α3, shows no ivermectin binding and is ≈4,000-fold resistant (6). Ivermectin also activates insect GluCl channels, and it is assumed that the effect on GluCl channels is at least partly responsible for ivermectin's insecticidal activity (5). However, effects of ivermectin on other ion channels are known and may contribute to the drug's efficacy. Most noticeably, ivermectin binds to and interacts with insect γ-aminobutyric acid (GABA)-gated chloride channels (7, 8). Together with glycine-gated channels, GABA-gated channels are the closest homologues of GluCl channels and are likely to be sites of ivermectin toxicity.
Because of its genetic accessibility, Drosophila is a valuable model system. In this study, Drosophila was used to examine the mode of action of the novel insecticide nodulisporic acid. A Drosophila strain resistant to nodulisporic acid was selected in the laboratory and examined for the molecular mechanism of resistance. Head membranes prepared from the resistant strain had lower affinity for nodulisporic acid and ivermectin than wild-type head membranes. A combination of genetics and sequencing identified a point mutation in the gene coding for the glutamate-gated chloride channel subunit DmGluClα. Electrophysiological studies of wild-type and mutant channels expressed in Xenopus oocytes revealed that nodulisporic acid directly activates DmGluClα and that the mutation (P299S) renders the channel less sensitive to activation by nodulisporic acid, ivermectin, and the endogenous ligand glutamate.
Materials and Methods
Fly Culture and Maintenance.
Flies were cultured as described (9, 10) at 25°C, 65% humidity, and 12-h light:12-h dark cycle. Unless insecticides were added to the culture, flies were raised on standard cornmeal, yeast, sucrose, glucose, and agar medium. Fly food containing insecticides was made with Instant Drosophila medium, formula 4-24 (Carolina Biological Supply), to which a mixture of water and insecticide dissolved in DMSO was added. The following Drosophila strains were used: wild-type Oregon-R, Or-R; white eyed wild-type, w; Resistance to dieldrin, RdlMD-RR (A302S); a second and third chromosome balancer, SM6aCyO; TM6B, Tb/apxa; a second chromosome balancer, SM6b, CyO/Sp J L Pin; a third chromosome balancer, TM3,Ser e/TM6B,Tb e (all provided by the Bloomington Drosophila Stock Center, Bloomington, IN); a glc deficiency line Df(3R)ora12/TM6, Ubx, referred to as Df12, which contains breakpoints 92A6:92D2; a deficiency line Df(3R) oraB16 adjacent to Df12, referred to as DfB16, which contains breakpoints 92A12–92B1; and 92E10–92E15 (11, 12). glc1, derived from Or-R by nodulisporic acid selection pressure, refers to nodulisporic acid- and ivermectin-resistant glc1/glc1.
Toxicity Assays.
For larval lethal assays, approximately 40 egg-laying adults were placed in a vial of food containing various doses of drug and allowed to lay eggs for 5 days, after which adults were removed. The vials were monitored for 3 wk after adult removal for the development of larvae, pupae, and adults. If one of the parental genotypes was not able to survive on drugged food for 5 days, eggs and first instar larvae were collected in a drug-free vial and then transferred to the drugged vial. The dose of drug at which no adults emerged was considered the larval LD100.
Binding Assays.
Drosophila head membranes were prepared, and binding of radiolabeled nodulisporic acid and ivermectin was measured as described (3), with the modification that both tritiated and 35S-labeled compounds were used. All radiolabeled compounds were synthesized at Merck Research Laboratories. [3H]Nodulisporic acid (22 Ci/mmol) refers to the tritiated amide derivative N-(2-hydroxyethyl-2,2-3H) nodulisporamide (3); [35S]nodulisporic acid (1,000 Ci/mmol) is a disubstituted amide derivative, N-1-[(4-35SO2Me)piperizinyl] nodulisporamide (13); and [3H]ivermectin (44 Ci/mmol) and [35S]ivermectin (1,000 Ci/mmol) were synthesized as described (14, 15). Binding data were fit to the equation B = (Bmax⋅L)/(Kd + L), where B is the concentration of specifically bound ligand, Bmax is the maximal number of binding sites, L is the free ligand concentration, and Kd is the equilibrium dissociation constant. All assays were performed in triplicate and usually varied by <3%. Results obtained with tritiated and 35S-labeled compounds were comparable, and average data include experiments with both ligands.
Genetic Mapping of Nodulisporic Acid Resistance.
Chromosomes X, II, and III from glc1 flies were tested for the presence of the nodulisporic acid resistance gene by a series of outcrosses (10–15 lines for each chromosome) with balancer stocks (15, 16). Once nodulisporic acid resistance had been mapped to chromosome III, deficiency mapping was performed with the line Df12, known to contain a deletion at the glc locus. Male glc1 were crossed to Df12 virgin females, and the progeny were tested in toxicity assays.
In Situ Hybridization.
In situ hybridization of larval salivary polytene chromosomes from Or-R, Df12, and DfB16 was performed as described previously (17) by using a DmGluClα cDNA probe (GenBank accession no. U58776) (5). DmGluClα in pBluescript (Stratagene) was linearized by BamHI, and random-prime labeled with digoxigenin (DIG), by using a DIG DNA Labeling and Detection Kit (Boehringer Mannheim). The labeled DNA was diluted to a concentration of 2 ng/μl in 5 × SSC/0.1% SDS, and stored at −20°C before use. Chromosome squashes were prepared by standard procedures (17). The chromosome slides were heated to 80°C in 2× SSC for 40 min. The chromosomes were denatured in 0.1 M NaOH for 2 min, rinsed in 2× SSC for 2 min, dehydrated through ethanol washes: twice for 2 min in 70% ethanol, once for 5 min in 95% ethanol, and air dried. The DIG-labeled probe was denatured, and 30 μl/slide was applied. The slides were sealed under coverslips with rubber cement. After overnight incubation at 65°C, the coverslips were washed in 2× SSC for 3 min. The slides were further washed three times for 2 min in PBT (PBS/0.1% Tween 20). Anti-DIG-alkaline phosphatase antibody of the DNA Detection kit was diluted 1:200 in PBT and incubated with the chromosomes (50 μl/slide) for 1.5 h at room temperature. The slides were then washed three times for 1 min in PBT and three times for 1 min in X-phosphate buffer (100 mM NaCl/50 mM MgCl2/100 mM Tris⋅HCl, pH 9.0/0.1% Tween 20), and the hybridization signal was developed by using the DNA detection kit. The chromosomes were counterstained in 1:20 dilution of Giemsa (Sigma) for 10 min, destained in water, air dried, and mounted with Permount (Sigma).
Behavioral Tests.
For spontaneous locomotion tests, individual flies were placed in a cylindrical chamber (8 mm diameter × 3 mm high), and the number of times each fly crossed the midline in a given amount of time was recorded. At least 20 7-day-old males of each genotype were tested at the same time of day for a 4-min period. Locomotion data were reported as quantiles and medians, and nonparametric statistical tests (Wilcoxon/Kruskal-Wallis rank sums) were performed by using JMP 3.2.2 (SAS Institute, Cary, NC) software. P values of <0.01 were considered significant (9). Bang sensitivity was measured by placing flies in a vial, vortexing for 5 s, and timing the rate of recovery from paralysis (18, 19). Temperature sensitivity was determined by placing 10 flies in a humidified glass vial immersed in a 37°C water bath for 30 min. For all behavioral tests, flies were transferred by aspiration rather than CO2 anesthetization and allowed to recover from aspiration for 10 min before testing.
Sequencing.
The DmGluClα cDNA was obtained from nodulisporic acid-resistant glc1 flies by reverse transcriptase PCR (RT-PCR). Total RNA was prepared from 10 glc1 flies (Ultraspec RNA; Biotecx Laboratories, Houston), and first strand synthesis of cDNA was performed by using oligo(dT) (GIBCO/BRL Superscript Preamplification System). The total first strand cDNA was used as template in six parallel PCR reactions, with primers based on wild-type DmGluClα: 5′-ATG GGC AGC GGA CAC TAT TTC and 3′-TTA CTC ATC CTC CTC CTC CCT GAAG or 3′-TTC GAA CCT GAT CGG GAT. The resulting 1,370- or 1,009-bp PCR products were purified (Gene Clean II; Bio 101), cloned into 2.1-Topo TA vector (Invitrogen) and transformed into DH5α electrocompetent Escherichia coli (GIBCO/BRL). The DNA sequence of 1–2 clones originating from each of six PCR reactions was determined. The GenBank/EBI Data Bank accession numbers for these cDNAs are AF297500 and AF297501.
Mutagenesis and in Vitro RNA Synthesis.
RNA coding for DmGluClα wild-type and P299S (C1299T) mutant channels was synthesized for injection into Xenopus oocytes. Wild-type DmGluClα (GenBank/EBI Data Bank U58776) was subcloned into pBluescript (Stratagene) to generate single-stranded DNA template, and site-directed mutagenesis was performed by using the Sculptor in vitro mutagenesis system (Amersham) and custom-designed mutagenic primers (GIBCO/BRL, 5′-GTC GGG CAT AAA CGC CTC CCT GTC GCC CGT TTC CTA TAC GAAG) according to the manufacturer's protocol. A T7 promoter and a poly(A+) tail were added to wild-type and mutant DmGluClα by PCR, and the resulting PCR products were used as templates in the in vitro transcription reaction (mMessage mMachine; Ambion, Austin, TX).
Electrophysiology in Xenopus laevis Oocytes.
Wild-type and P299S RNAs were injected into X. laevis oocytes for electrophysiological analysis. Oocytes were prepared as previously described (20) and studied by two-electrode voltage clamp 1–4 days after injection with 50 nl of 100–200 ng/μl RNA. Whole cell currents were measured by using a Dagan (Minneapolis) CA-1 amplifier interfaced to a Macintosh 7100/80 computer. Data were acquired at 100 Hz by using Pulse software (HEKA Electronics, Lambrecht/Pfalz, Germany). During recording, oocytes were continuously superfused with a solution containing 96 mM NaCl, 0.1 mM CaCl2, 1 mM MgCl2, 3.5 mM BaCl2, and 5 mM Hepes (pH 7.5). Drugs were dissolved in DMSO and applied in the recording solution at ≤0.1% DMSO. All experiments were performed at room temperature from a holding potential of 0 mV. Currents activated by ivermectin or nodulisporic acid were normalized to the maximal glutamate-induced current in the same oocyte, and dose-response curves were fit to the Hill equation with a Hill coefficient of 2: I = Imax/(1 + (EC50/L)2), where L is the concentration of drug and Imax is the current activated by saturating drug concentrations.
Results
Toxicity Assays.
Nodulisporic acid-resistant flies were generated from Or-R by growth for three generations on 0.29 μM nodulisporic acid, where the larval LD100 = 0.44 μM, followed by stepwise increases in the selection pressure over approximately 20 generations to a final concentration of 10.3 μM nodulisporic acid (Table 1). The resulting strain of flies, called glc1, was >23-fold resistant to nodulisporic acid. The heterozygote glc1/+ shows intermediate resistance with an LD100 of 1.5 μM. In addition, glc1 flies are at least 3.3-fold cross-resistant to ivermectin with larval LD100 of 0.12 μM for glc1 and 0.035 μM for Or-R. In contrast, glc1 flies showed no differences in sensitivity to fipronil; the fipronil larval LD100 for both Or-R and glc1 was 0.57 μM. As with other drugs, adult flies were less sensitive to nodulisporic acid, ivermectin, and fipronil than larvae (data not shown). Because ivermectin is known to interact with GABA receptors in vertebrates and nematodes (for examples, see refs. 21 and 22), Drosophila carrying mutations in a GABA-gated channel were tested for drug sensitivities. RdlMD-RR flies contain an alanine to serine mutation in the second transmembrane domain of the Rdl gene coding for a GABA-gated channel and are >4000-fold resistant to the insecticide dieldrin (23). RdlMD-RR flies are approximately 3.3-fold resistant to ivermectin, 5-fold resistant to nodulisporic acid, and 6-fold resistant to fipronil.
Table 1.
Larval LD100 for the insecticides nodulisporic acid, ivermectin, and fipronil on Drosophila strains Or-R, RdlMD-RR, glc1, glc1/+, glc1/Df12, and Df12/+
| Nodulisporic acid, μM | Ivermectin, μM | Fipronil, μM | |
|---|---|---|---|
| Or-R | 0.44 | 0.03 | 0.57 |
| n = 6 | n = 4 | n = 2 | |
| RdlMD-RR | 2.20 | 0.12 | 3.43 |
| n = 4 | n = 3 | n = 3 | |
| glc1 | >10.3* | 0.12 | 0.57 |
| n = 3 | n = 2 | n = 2 | |
| glc1/+ | 1.50 | ||
| n = 3 | |||
| glc1/Df12 | >5.9* | ||
| n = 5 | |||
| Df12/+ | 0.44 | ||
| n = 3 |
Numbers represent the larval LD100; n equals the number of trials.
Highest concentration of nodulisporic acid tested.
Drug Binding to glc1 Drosophila Membranes.
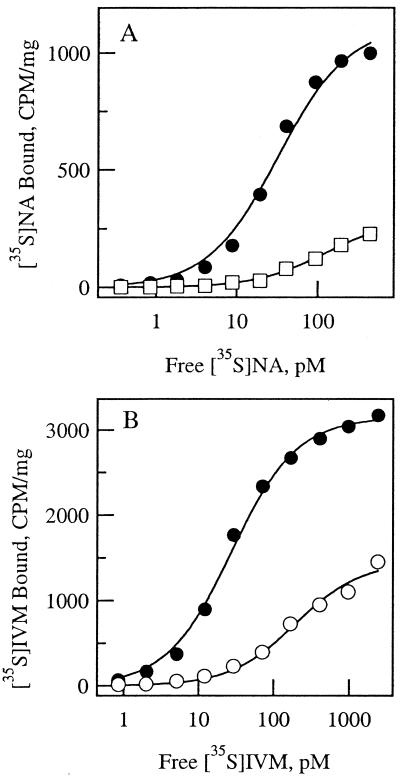
The nodulisporic acid- and ivermectin-resistant glc1 flies were tested for changes in the binding affinities of radiolabeled drug to head membrane receptors. [35S]Nodulisporic acid binds with high affinity and in a saturable manner to Drosophila Or-R head membranes (Fig. 1A). Fitting of the data to a simple mass action binding isotherm yielded Kd= 33 pM and Bmax = 1.1 pmol/mg protein. [35S]Nodulisporic acid binding to head membranes of glc1 flies was of considerably lower affinity and was near saturation at 400 pM, the highest concentration tested. Least squares fitting of the data according to the law of mass action yielded Kd = 140 pM and Bmax= 0.27 pmol/mg protein. In four experiments, [35S]nodulisporic acid binding affinity was shifted a geometric mean of 7-fold in glc1 flies compared with wild-type flies. Because glc1 flies showed 3.3-fold cross-resistance to ivermectin (Table 1), [35S]ivermectin binding was examined in head membranes of wild-type and glc1 flies (Fig. 1B). [35S]Ivermectin binding to wild-type membranes was saturable, with Kd = 26 pM and Bmax= 2.7 pmol/mg. For the glc1 flies, fitting of the data according to the law of mass action was in agreement with binding to a single site, with Kd= 190 pM and Bmax= 1.4 pmol/mg. Although the existence of multiple sites cannot be ruled out, no population of sites was seen at the Kd for [35S]ivermectin binding to wild-type membranes. Therefore, all ivermectin binding sites of head membranes appear to be affected in the resistant strain, and the average reduction in binding affinity was 7-fold (n = 7), the same as for nodulisporic acid.
Figure 1.
Nodulisporic acid and ivermectin binding to wild-type and glc1 Drosophila head membranes. (A) Specific binding of [35S]nodulisporic acid to wild-type (filled symbols) and glc1 (open symbols, two separate experiments) Drosophila head membranes. Specific binding was defined as total binding, measured in triplicate, minus binding in the presence of 100 nM unlabeled nodulisporic acid. Data were fit to the binding isotherm of the form: B = (Bmax⋅L)/(Kd + L), where B is the concentration of specifically bound ligand, Bmax is the maximal number of binding sites, L is the free ligand concentration, and Kd is the equilibrium dissociation constant. (B) Specific binding of [35S]ivermectin to wild-type (filled symbols) and glc1 (open symbols, two separate experiments) Drosophila head membranes.
Genetic Mapping.
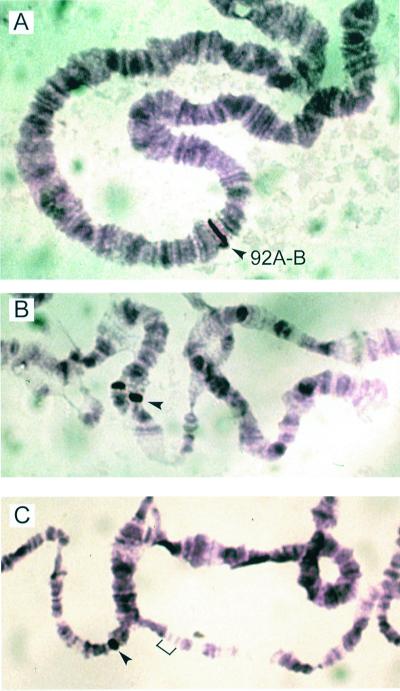
The DmGluClα gene location was examined by using in situ hybridization. A single hybridization band was detected at cytological position 92A-B on chromosome 3 of wild-type Or-R third instar larvae (Fig. 2A). This site was mapped further by in situ hybridization to deficiency chromosomes DfB16 and Df12 (11). The DmGluClα probe hybridized to DfB16 (Fig. 2B) but not to Df12 (Fig. 2C); therefore, the DmGluClα gene is located on chromosome 3, between the breakpoints of Df12 and DfB16 (92A4–92B2).
Figure 2.
In situ hybridization of DmGluClα probe to cytological position 92A-B on Or-R polytene chromosome 3 (A). The DmGluClα probe hybridizes to heterozygous deficiency chromosome DfB16 (B) but not Df12 (C).
Standard linkage analysis techniques mapped the nodulisporic acid resistance in glc1 flies to chromosome 3. Like homozygous glc1/glc1 flies, Df12/glc1 flies were more than 10-fold resistant to nodulisporic acid, whereas Df12/+ exhibited the same LD100 as Or-R (Table 1). These data suggest that the Df12 deletion, spanning the region 92A6–92D2 and containing DmGluClα, includes the site of the resistance gene.
Behavioral Phenotype of Drug-Resistant Drosophila.
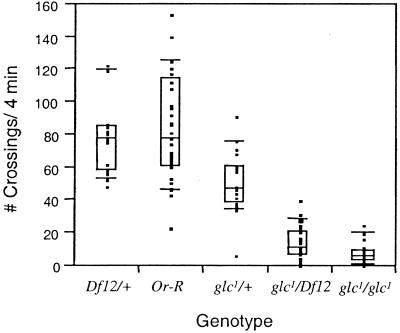
Compared with wild type, glc1 flies are less vigorous, as evidenced by impaired locomotion and flight ability and a greatly reduced brood size. A quantitative measure of reduced locomotion is shown in Fig. 3. glc1 and glc1/Df12 flies exhibited significantly reduced locomotion, compared with Or-R and Df12/+ (P < 0.0001) and were not significantly different from each other (P > 0.02). During a period of 4 min, glc1 and glc1/Df12 flies crossed the center line a median of 5.5 times and 11 times, respectively, compared with 78 times for Or-R and Df12/+. The heterozygotes, glc1/+, exhibited an intermediate level of locomotion of 47 crosses/4 min, significantly different from all other genotypes (P < 0.0001). These phenotypes combined with the moderate resistance of the heterozygotes to nodulisporic acid (Table 1) suggest that the glc1 mutation is semidominant. None of the genotypes tested were temperature sensitive, and only glc1 and glc1/Df12 were bang sensitive, exhibiting significantly more paralysis than Or-R after 5 s of vortexing.
Figure 3.
Spontaneous Locomotion. Twenty to thirty-three flies of specified genotypes were tested in spontaneous locomotion assays. Data are presented as medians and quantiles, where the center lines indicate medians, boxes represent the 25% and 75% quantiles, bars the 10% and 90% quantiles, and black squares are the individual data points.
Molecular Analysis.
The molecular nature of nodulisporic acid resistance in glc1 flies was further examined by direct analysis of DmGluClα cDNAs. Several DmGluClα cDNAs were obtained from glc1 flies by reverse transcription, followed by six separate PCR reactions to control for artifacts. Nine DmGluClα cDNA clones from glc1 flies were analyzed, and all contained a point mutation resulting in a proline to serine change (P299S). The proline residue P299 is ubiquitous in all known glutamate-, GABA-, and glycine-gated chloride channels and is located immediately C-terminal to the M2 domain. In addition to the P299S mutation, sequencing revealed alternative splicing and RNA editing in DmGluClα, also documented by Semenov and Pak (24). Therefore, in glc1 flies, a mixture of alternatively spliced and edited DmGluClα mRNAs appeared to be expressed, all containing the P299S mutation.
Nodulisporic Acid Activation of DmGluClα.
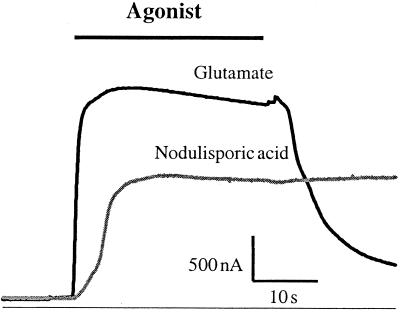
The effects of nodulisporic acid on recombinant DmGluClα channels were examined in Xenopus oocytes. Fig. 4 shows representative glutamate- and nodulisporic acid-activated currents from an oocyte expressing DmGluClα. The nodulisporic acid-sensitive current had slower activation kinetics than the glutamate-sensitive current and was essentially irreversible on wash-out of the drug. In the experiment shown, saturating concentrations of nodulisporic acid activated approximately 60% of the maximum glutamate-activated current. The relative current amplitudes varied somewhat between experiments, and values between 30% and 60% were observed, with average value of 43 ± 12% (n = 5).
Figure 4.
Nodulisporic acid activates DmGluClα. An oocyte expressing DmGluClα exhibited a rapidly activating, reversible current in response to application of 100 μM glutamate. In the same oocyte, 1 μM nodulisporic acid activated a current that was essentially irreversible on wash-out of nodulisporic acid and measured 60% of the glutamate-activated current.
Electrophysiology of DmGluClα P299S.
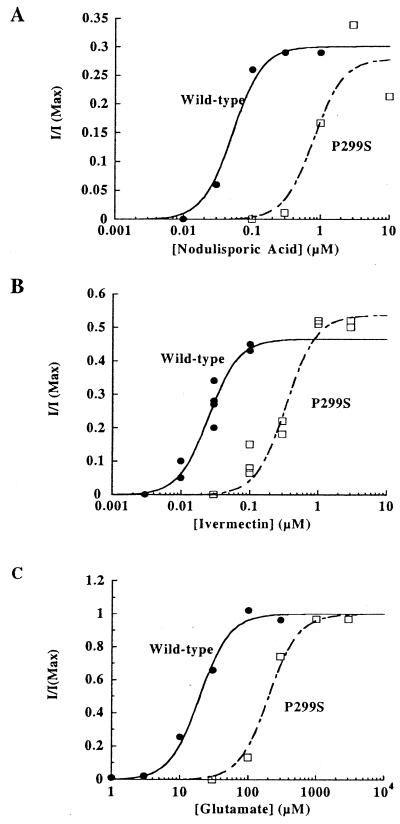
To further test the hypothesis that the activity of nodulisporic acid and ivermectin on glutamate-gated chloride channels was the basis for their insecticidal properties, DmGluClα P299S was constructed by site-directed mutagenesis. Wild-type and P299S DmGluClα were expressed in Xenopus oocytes and examined by the two-electrode voltage clamp method. Oocytes injected with either wild-type or P299S DmGluClα RNA produced similar currents in response to high concentrations of glutamate; however, glutamate dose-response curves revealed a reduced sensitivity to glutamate in P299S, as compared with wild-type (Fig. 5C). Fitting the data to the Hill equation (nh = 2) yielded EC50 = 19.5 μM for wild-type and 201 μM for P299S channels. Nodulisporic acid and ivermectin activated wild-type and mutant channels irreversibly; therefore, dose-response curves were generated by normalizing nodulisporic acid- and ivermectin-activated currents to the current observed with saturating glutamate concentrations. The maximum current activated by nodulisporic acid was similar for wild-type and P299S channels and in separate experiments reached 30–60% of the maximal glutamate-activated current. However, DmGluClα P299S showed greatly reduced sensitivity to nodulisporic acid (Fig. 5A). EC50 were 51 nM for wild-type and 790 nM for P299S. This observed 15-fold reduction in nodulisporic acid sensitivity strengthens the hypothesis that, similar to ivermectin, nodulisporic acid acts by activating DmGluCl channels. Finally, responses of wild-type and P299S channels to ivermectin were examined. As for glutamate and nodulisporic acid, the dose-response curve for ivermectin was right-shifted in P299S, yielding an EC50 of 340 nM compared with 25 nM for DmGluClα wild-type (Fig. 5B). This approximately 14-fold reduction in ivermectin sensitivity on the level of the DmGluClα channel was greater than expected from the ivermectin cross-resistance seen in glc1 flies and was similar to the reduction in nodulisporic acid sensitivity.
Figure 5.
Agonist sensitivity of DmGluClα P299S. Dose-response curves for nodulisporic acid (A), ivermectin (B), and glutamate (C) activation of DmGluClα wild-type (filled circles) and P299S (open squares) channels. Oocytes injected with DmGluClα wild-type or P299S RNA were superfused with varying drug concentrations, and currents were normalized to the current activated by saturating concentrations of glutamate. Data were fit to the Hill equation with nh = 2.
Discussion
Using a drug-resistant strain of Drosophila and oocyte expression of recombinant channels, we have shown that glutamate-gated chloride channels are a target for the novel insecticide nodulisporic acid and substantiated this receptor as a target for ivermectin. Glc1 flies resistant to nodulisporic acid carry a single point mutation in DmGluClα. This change from a highly conserved proline, located distal to the second transmembrane domain, to a serine residue was found in all nine glc1 DmGluClα cDNAs sequenced. Although we cannot rule out the possibility that additional mutations within the region 92A6–92D2 of chromosome 3 may contribute to nodulisporic acid resistance, the properties of recombinant mutant and wild-type channels are consistent with this point mutation underlying the major site of nodulisporic acid resistance seen in glc1 flies. Expressed in oocytes, the P299S mutation rendered recombinant DmGluClα channels >10-fold less sensitive to activation by a number of ligands, including nodulisporic acid and ivermectin. That the mutant DmGluClα channel found in glc1 flies was significantly less sensitive to activation by the endogenous ligand glutamate may be the reason for the reduced viability and the sickly phenotype of glc1 flies. This hypothesis was supported by the fact that glc1 flies appeared healthier when kept on nodulisporic acid than in the absence of any drug (unpublished results). Like ivermectin, nodulisporic acid potentiated the response of DmGluClα channels to glutamate at concentrations less than needed for channel activation (data not shown); therefore, sublethal levels of nodulisporic acid may restore some of the glutamate-dependent neurotransmission in glc1 flies.
On a molecular level, the P299S mutation further highlights the significance of the M2-M3 linker region in ligand-gated chloride channels. Resistance of Drosophila to the insecticides dieldrin and fipronil is due to a point mutation in the M2 region of GABA-gated channels (23, 25). In human glycine-gated chloride channels, a point mutation in the extracellular terminus of the M2 segment results in hyperekplexia, known as startle disease, which is a dominant neurological disorder. People with hyperekplexia exhibit increased neural hyperexcitability, probably as a result of decreased agonist affinities (26). In addition, residues in the M2-M3 linker region are critical to volatile anesthetic response in both glycine- and GABA-gated chloride channels (27).
The physiological function of the DmGluClα gene remains unclear. The bang-sensitive and lethargic phenotype of the glc1 flies suggests neurologic defects. The map location of DmGluClα and glc1 drug resistance on chromosome 3;92A4–92B corresponds to a region known to play a role in the visual system. The ora transientless or ort gene maps to position 92B1, and mutations in this gene lead to visual behavior-defective phenotypes (11, 28). Electroretinograms obtained from ort mutants lack the membrane potential transients typically seen at the on- and offset of a light stimulus (11). Because these transients originate from cells in the lamina (29), it was suggested that the ort gene may play a role in photoreceptor signaling (11). Several lines of evidence point to histamine as the major neurotransmitter in Drosophila photoreceptors (30), and it has been postulated that the ort gene may encode a histamine-gated ion channel (W. L. Pak, personal communication). The DmGluClα homomultimer channel as expressed in oocytes does not respond to histamine (5); however, the Drosophila genome sequence now reveals another predicted ligand-gated channel at position 92B1, which may be the candidate for the ort gene (28).
Glutamate-gated chloride channels have been found only in invertebrates, making them a safe target for anthelmintic and insecticidal drugs. Ivermectin activates glutamate-gated chloride channels, and the ivermectin cross-resistance of glc1 flies carrying a mutation in the DmGluClα gene further suggests that this mode of action contributes to ivermectin's insecticidal activity. Ivermectin is widely used to control parasites in humans and animals; however, some cases of ivermectin toxicity have been observed in collies and CF-1 mice, suggesting that ivermectin can act on targets in mammalian brain (31, 32). Possible candidates are mammalian GABA-gated and glycine-gated ion channels, the closest mammalian homologues of invertebrate GluCl channels. In fact, there are a number of reports of ivermectin binding to GABAA and glycine receptors and activation of GABAA receptors in rodent brain (21, 33, 34). Binding studies on Drosophila head membranes suggest that nodulisporic acid binds only to a subset of ivermectin binding sites with high affinity (3). Hopefully, this greater specificity of nodulisporic acid will be associated with reduced toxicity.
The mutation found in glc1 flies confers greater resistance to nodulisporic acid than to ivermectin. There are two possible explanations for this phenomenon. It may be that the P299S mutation has differential effects on ivermectin and nodulisporic acid actions at the same receptor, which is not the case for the recombinant DmGluClα homomultimer expressed in oocytes but cannot be completely ruled out for the native receptor. Alternatively, nodulisporic acid-insensitive ivermectin receptors may be less affected than nodulisporic acid-sensitive ivermectin receptors. Considering the strong effect of the P299S mutation on ivermectin activation of DmGluClα homomultimers, one could postulate the presence of additional unidentified subunits in native nodulisporic acid-insensitive ivermectin receptors.
Recent immunoprecipitation studies suggest that Drosophila nodulisporic acid receptors contain both glutamate-gated (DmGluClα) and GABA-gated (Rdl) chloride channel subunits (S.W.L., unpublished results). In support of these results, flies carrying a mutation in the Rdl gene were 5-fold resistant to nodulisporic acid. In the same studies, all of the solubilized nodulisporic acid and ivermectin binding sites could be immunoprecipitated with antibodies to DmGluClα. A mutation in DmGluClα, as is found in the nodulisporic acid-resistant Drosophila strain glc1, would therefore be expected to affect all nodulisporic acid and ivermectin binding sites, in agreement with the binding data presented in this study.
Indirect evidence from binding studies and electrophysiological recordings in grasshopper neurons has pointed to glutamate-gated chloride channels as a target for the novel insecticide nodulisporic acid. The direct activation of the DmGluClα channel and the DmGluClα P299S mutation found in nodulisporic acid-resistant Drosophila strongly support this mode of action. In addition, the P299S mutation is a useful tool in mapping sites on ligand-gated ion channels that play a role in channel activation.
Acknowledgments
We thank Dr. Michel Hamelin, Dr. Greg Kaczorowski, and Michael Cuddy for helpful discussions; Dr. Peter Meinke (medicinal chemistry) and Dr. Dennis Dean (labeled compound synthesis) for the radiolabeled ligands; and Dr. Jeff Yuan for assistance in bioinformatics. We also thank the Indiana Stock Center and Dr. William Pak for providing Drosophila stocks.
Abbreviations
- glc
GluClα
- GABA
γ-aminobutyric acid
- DIG
digoxigenin
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF297500 and AF297501).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240464697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240464697
References
- 1.Ostlind D A, Felcetto T, Misura A, Ondeyka J, Smith S, Goetz M, Shoop W, Mickle W. Med Vet Entomol. 1997;11:407–408. doi: 10.1111/j.1365-2915.1997.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 2.Ondeyka J G, Helms G L, Hensens O D, Goetz M A, Zink D L, Tsipouras A, Shoop W L, Slayton L, Dombrowski A W, Polishook J D, et al. J Am Chem Soc. 1997;119:8809–8816. [Google Scholar]
- 3.Smith M M, Warren V A, Thomas B S, Brochu R M, Ertel E A, Rohrer S, Schaeffer J, Schmatz D, Petuch B R, Tang Y S, et al. Biochemistry. 2000;39:5543–5554. doi: 10.1021/bi992943i. [DOI] [PubMed] [Google Scholar]
- 4.Cully D F, Vassilatis D K, Liu K K, Paress P S, Van der Ploeg L H, Schaeffer J M, Arena J P. Nature (London) 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 5.Cully D F, Paress P S, Liu K K, Schaeffer J M, Arena J P. J Biol Chem. 1996;271:20187–20191. doi: 10.1074/jbc.271.33.20187. [DOI] [PubMed] [Google Scholar]
- 6.Dent J A, Smith M M, Vassilatis D K, Avery L. Proc Natl Acad Sci USA. 2000;97:2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abalis I M, Eldefrawi A T. Pestic Biochem Physiol. 1986;25:279–287. [Google Scholar]
- 8.Bermudez I, Hawkins C A, Taylor A M, Beadle D J. J Recept Res. 1991;11:221–232. doi: 10.3109/10799899109066401. [DOI] [PubMed] [Google Scholar]
- 9.Kane N S, Robichon A, Dickinson J A, Greenspan R J. Neuron. 1997;18:307–314. doi: 10.1016/s0896-6273(00)80270-6. [DOI] [PubMed] [Google Scholar]
- 10.Griffith L C, Verselis L M, Aitkin K M, Kyriacou C P, Danho W, Greenspan R J. Neuron. 1993;10:501–509. doi: 10.1016/0896-6273(93)90337-q. [DOI] [PubMed] [Google Scholar]
- 11.O'Tousa J E, Leonard D S, Pak W L. J Neurogenet. 1989;6:41–52. doi: 10.3109/01677068909107099. [DOI] [PubMed] [Google Scholar]
- 12.Wilson J E, Connell J E, Schlenker J D, Macdonald P M. Dev Genet. 1996;19:199–209. doi: 10.1002/(SICI)1520-6408(1996)19:3<199::AID-DVG3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Meinke P T, Ayer M B, Coletti S L, Li C, Lim J, Ok D, Salva S, Schmatz D M, Shih T L, Shoop W L, et al. Bioorg Med Chem Lett. 2000;10:2371–2374. doi: 10.1016/s0960-894x(00)00469-8. [DOI] [PubMed] [Google Scholar]
- 14.Schaeffer J M, Haines H W. Biochem Pharmacol. 1989;38:2329–2338. doi: 10.1016/0006-2952(89)90473-5. [DOI] [PubMed] [Google Scholar]
- 15.Meinke P T, Rohrer S P, Hayes E C, Schaeffer J M, Fisher M H, Mrozik H. J Med Chem. 1992;35:3879–3884. doi: 10.1021/jm00099a015. [DOI] [PubMed] [Google Scholar]
- 16.Roberts D B. In: The Practical Approach. Hames B D, editor. Oxford: IRL; 1998. p. 389. [Google Scholar]
- 17.Greenspan R J. Fly Pushing: The Theory and Practice of Drosophila Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [Google Scholar]
- 18.Ashburner M. Drosophila: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 19.Jan Y N, Jan L Y. Proc Natl Acad Sci USA. 1978;73:515–519. doi: 10.1073/pnas.75.1.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganetzky B, Wu C. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Casida J E. J Pharmacol Exp Ther. 1997;281:261–266. [PubMed] [Google Scholar]
- 22.Martin R J, Pennington A J. Br J Pharmacol. 1989;98:747–756. doi: 10.1111/j.1476-5381.1989.tb14602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ffrench-Constant R H, Rocheleau T A, Steichen J C, Chalmers A E. Nature (London) 1993;363:449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- 24.Semenov E P, Pak W L. J Neurochem. 1999;72:66–72. doi: 10.1046/j.1471-4159.1999.0720066.x. [DOI] [PubMed] [Google Scholar]
- 25.Hosie A M, Baylis H A, Buckingham S D, Sattelle D B. Br J Pharmacol. 1995;115:909–912. doi: 10.1111/j.1476-5381.1995.tb15896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langosch D, Laube B, Rundstrom N, Schmieden V, Bormann J, Betz H. EMBO J. 1994;13:4223–4228. doi: 10.1002/j.1460-2075.1994.tb06742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihic S J, Ye Q, Wick M J, Kolchine V V, Krasowski M D, Finn S E, Mascia M P, Valenzuela C F, Hanson K K, Greenblatt E P, et al. Nature (London) 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 28.The FlyBase Consortium. Nucleic Acids Res. 1999;27:85–88. [Google Scholar]
- 29.Coombe P E. J Comp Physiol. 1986;159:655–665. [Google Scholar]
- 30.Stuart A E. Neuron. 1999;22:431–433. doi: 10.1016/s0896-6273(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 31.Paul A J, Tranquilli W J, Seward R L, Todd K S J, DiPietro J A. Am J Vet Res. 1987;48:684–685. [PubMed] [Google Scholar]
- 32.Lankas G R, Cartwright M E, Umbenhauer D. Toxicol Appl Pharmacol. 1997;143:357–365. doi: 10.1006/taap.1996.8086. [DOI] [PubMed] [Google Scholar]
- 33.Kamijima M, Casida J. Appl Pharmacol. 2000;163:188–194. doi: 10.1006/taap.1999.8865. [DOI] [PubMed] [Google Scholar]
- 34.Krusek J, Zemkova H. Eur J Pharmacol. 1994;259:121–128. doi: 10.1016/0014-2999(94)90500-2. [DOI] [PubMed] [Google Scholar]