Abstract
Enhancement of coronary collateral function is an intriguing approach to the preservation of ischaemic myocardium. Coronary collateral development consists of collateral recruitment and collateral growth. Collateral growth encompasses proliferation of capillaries in the ischaemic area (angiogenesis) and maturation of pre-existing collateral vessels (arteriogenesis), with the latter being more relevant in humans. Therefore, treatment intended directly for arteriogenesis of collateral vessels appears to be more effective. Promotion of coronary collateral growth has many attractive features, particularly in patients with angina who are not indicated for percutaneous coronary intervention or coronary artery bypass grafting surgery. A complete elucidation of the remaining practical and mechanistic questions of arteriogenesis may lead to a new remedy capable of developing collateral vessels more effectively.
Keywords: coronary collaterals, ischaemia, angiogenesis, arteriogenesis
A new treatment option to conventional coronary interventions is angiogenic treatment, which is defined as enhancement of collateral function by acute or chronic administration of some agents for the preservation of ischaemic myocardium.1,2 Augmentation of collateral circulation is achieved by collateral recruitment or arteriogenesis. Collateral recruitment means that pre-existing collateral vessels increase their lumen by passive dilatation so as to enhance collateral flow to the jeopardised ischaemic regions. Arteriogenesis of collateral vessels means that the lumen of pre-existing collateral arterioles increases with proliferation of vascular smooth muscle cells so as to provide enhanced perfusion to the collateral dependent ischaemic regions.3–5 Angiogenesis, the proliferation, migration, and tube formation of capillaries in the central area of ischaemic regions, is a different concept from collateral development.3–5 Until 10 years ago, methods for assessing collateral function were limited to surrogate markers of collateral flow—that is, parameters of regional myocardial ischaemia and angiographic visualisation of collateral channels, which is a poor indicator of collateral flow. The advent of intracoronary pressure or Doppler wires has enabled assessment of collateral function more accurately and quantitatively.6–8 Although the importance of a well developed coronary collateral circulation is documented by numerous clinical reports,9,10 precise mechanisms for coronary collateral development remain to be clarified. Recent advances in molecular biology and genetic technology have provided some clues for defining the conditions and mechanisms for collateral development in the clinical setting. Moreover, the advent of angiogenic treatment for ischaemic patients who are not candidates for revascularisation procedures has shed light on the importance of collateral development. This review summarises the current knowledge of human coronary collateral development. Data defining the conditions and mechanisms for collateral development are also discussed.
CONDITIONS FOR COLLATERAL RECRUITMENT IN HUMANS
Well developed collateral vessels with several layers of muscular media change their vascular tone in response to various endogenous vasoactive substances and drugs. Collateral recruitment—progressive increase in collateral flow after the establishment of a pressure gradient across the collateral network—may account for walk-through angina in patients with a well developed collateral circulation. Cribier et al11 have shown that collateral flow is progressively augmented by repeated coronary artery occlusion by an angioplasty balloon. Glyceryl trinitrate is a potent vasodilator of coronary collateral vessels as well as of coronary conduit arteries.12 We observed that isosorbide dinitrate increased exercise capacity in patients with coronary artery disease only if collateral circulation was evident during coronary angiography.13 Aggregating platelets can release serotonin, which causes vasoconstriction of the collateral circulation. We reported that a serotonin blocker, sarpogrelate, had a positive effect, improving workload as well as myocardial perfusion (as measured by scintigraphy) in patients with good coronary collateral circulation, whereas it had no effect in patients without or with poor collateral circulation.14 Thus, administration of both nitrates and the serotonin blocker probably causes dilatation of collateral vessels and enhances blood flow to the collateral dependent myocardium. However, these findings should be confirmed by direct quantitative measurements of collateral circulation in future studies.
CONDITIONS FOR COLLATERAL GROWTH IN HUMANS
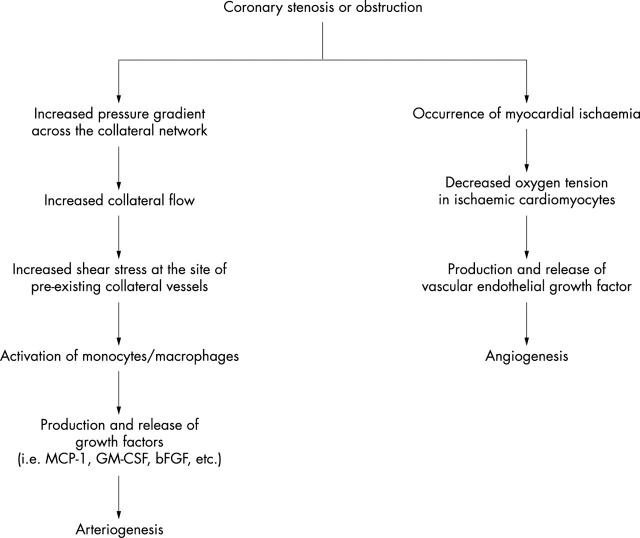
It is conceivable that a high grade gradual coronary stenosis can induce collateral vessel growth. Cohen et al15 found that a coronary stenosis of less than 80% luminal narrowing was rarely associated with angiographically demonstrable collateral circulation, whereas lesions of 95% or more narrowing nearly always were associated with good collateral filling after establishment of a pressure gradient across the collateral network by angioplasty balloon catheter. More recently, it has been shown that coronary lesion severity is the only independent variable related to collateral growth.16 A high grade coronary stenosis results in an increased transcollateral pressure gradient associated with increased collateral blood flow. Concomitantly, severe coronary stenosis provokes myocardial ischaemia in the area perfused by the culprit artery. Over the past decade, the relative importance for collateral growth of mechanical factors at the site of pre-existing collateral vessels and myocardial ischaemia have been investigated. Figure 1 presents a proposed mechanism of how severe coronary stenosis results in arteriogenesis and angiogenesis. A high grade coronary stenosis decreases the intra-arterial pressure at the distal site of the culprit lesion, resulting in an increased pressure gradient across the pre-existing collateral network. This change in the haemodynamic milieu causes increased collateral blood flow and resultant increased shear stress at the site of pre-existing collaterals. The increased shear stress develops collateral vessels through the promotion of angiogenic growth factors such as basic fibroblast growth factor (bFGF) produced by activated monocytes.3 A high grade coronary stenosis also provokes myocardial ischaemia, and ischaemic cardiomyocytes produce and release vascular endothelial growth factor (VEGF), which is a key substance for angiogenesis.17
Figure 1.
Mechanisms by which a high grade coronary stenosis causes arteriogenesis and angiogenesis. bFGF, basic fibroblast growth factor; GM-CSF, granulocyte macrophage colony stimulating factor; MCP-1, monocyte chemoattractant protein 1.
THERAPEUTIC MODULATIONS OF COLLATERAL GROWTH
Effort angina is frequently observed in patients with collaterals, suggesting that coronary flow reserve through the collateral circulation to the collateral dependent zone is limited. In these patients, treatment to promote collateral growth would be of merit in terms of quality of life and attenuation of the deleterious sequelae of coronary artery disease. Many attempts have been made to search for evidence that exercise training may enhance myocardial oxygen delivery by improving the coronary collateral circulation in patients with coronary artery disease. In a randomised trial, Nolewajka et al18 repeated coronary angiography to evaluate the changes in coronary collateral visualisation. However, angiographic evaluation of the coronary collateral circulation before and after the exercise programme showed no significant change. As already indicated, coronary angiography does not result in opacification of all collaterals and therefore the extent of collateralisation may have been underestimated. To clarify the benefits of an exercise programme on coronary collateral growth, Ehsani et al19 compared the double product at the time of 0.1 mV ST segment depression before and after a vigorous 12 month training programme. In fact, exercise training provided a 22% higher double product at 0.1 mV ST segment depression. They speculated that the increased double product necessary to provoke electrocardiographic evidence of myocardial ischaemia may suggest that myocardial oxygen delivery increases to keep supply and demand in balance. This speculation implicates the presence of further developed collateral vessels supplying the potentially ischaemic myocardium. It is desirable that future studies use more sensitive and direct techniques to analyse coronary collateral function; then a more accurate assessment of the beneficial effect of exercise training on the coronary collateral circulation will be possible. It is tempting to speculate about the underlying mechanisms by which intense exercise induces collateral growth in patients with severe coronary artery stenosis. Exercise increases metabolic demand in the collateral dependent myocardium, causing maximal dilatation in the resistance vessels. As a result, collateral blood flow increases and collateral growth is achieved with the cascade shown in fig 1.
The importance of heparin on collateral growth has been reviewed in detail.20 In the process of collateral growth shown in fig 1, heparin potentiates the action of angiogenic growth factors as described elsewhere.20 From this conceptual framework, the combined use of exercise and pharmacological agents may be proposed to augment collateral growth. In fact, the clinical effectiveness of combining heparin treatment with exercise stress has been reported and confirmed.21 Since there are many patients with intractable angina pectoris who are not candidates for percutaneous coronary intervention and coronary bypass surgery, the search for more treatment options for these patients is of great importance.
INDIVIDUAL DIFFERENCES IN COLLATERAL GROWTH
Although it is clear that a longstanding high grade coronary stenosis is responsible for collateral vessel growth,15,16 it remains unclear as to which factors affect the extent of collateral growth in the presence of severe coronary artery disease. Recently, we have reported that the extent of collateral growth is influenced largely by the age of patients.22 We evaluated the extent of angiographically demonstrable collateral vessels in patients with acute myocardial infarction with a history of longstanding effort angina. The prevalence of a well developed collateral circulation was significantly lower in the elderly group (39%, older than 65 years) than in the younger group (69%, younger than 65 years). In contrast, in a study of a large cohort of patients, age did not affect the degree of coronary collateral growth.16 The difference may be, at least in part, explained as follows. Firstly, we included only patients with a completely occluded collateral receiving vessel. Pohl et al16 studied patients with variable extents of stenosis in the collateral receiving vessel. Secondly, we used the Rentrop grading system of 0 to 3 based on angiographic opacification of the collateral receiving artery. On the other hand, Pohl et al16 assessed collateral function with intracoronary Doppler flow or pressure wires, or both.
Abaci et al23 have shown that patients with diabetes mellitus have a lesser ability to create collateral vessels. The authors speculated that endothelial dysfunction in diabetic patients may explain poorer collateral growth. Because the endothelium is one key element in the cascade of collateral growth (fig 1), the hypothesis raised by these authors appears to be reasonable. Waltenberger et al24 have recently documented that the ability of monocytes to migrate towards a gradient of VEGF-A is severely impaired in diabetic patients. In contrast, Melidonis et al25 have reported that diabetic patients develop more extensive coronary collateral circulation than non-diabetic patients. Furthermore, Pohl et al16 have shown that diabetic and non-diabetic patients have a comparable extent of coronary collateral development. The discrepant findings in these studies may be accounted for by the differences in the methods to evaluate coronary collateral function and the extent of stenosis of the collateral receiving artery.
Hypercholesterolaemia also impairs endothelial function; therefore, it is possible that the extent of collateral vessel growth is poorer in hypercholesterolaemia. Although attenuation of collateral vessel growth was clearly shown in the hind limb artery ligation model of the Watanabe heritable hyperlipidaemic rabbit,26 there are no clinical reports of a negative correlation between total plasma cholesterol concentration and collateral growth.
CORONARY COLLATERAL REGRESSION
Collateral regression involves two different concepts: functional and anatomical regression. Functional regression means that collateral vessels close after reperfusion of the recipient coronary artery, presumably due to the disappearance of collateral flow. It takes several minutes before pre-existing collateral vessels reopen completely after the re-establishment of a pressure gradient across the collateral network.27 Functional regression therefore depends on the interval between coronary occlusions. On the other hand, anatomical regression means that matured collateral vessels lose, in part, their well developed media and luminal size, and the conductance of collateral flow decreases despite a sufficient pressure gradient and time for collateral recruitment. Functional regression was reported in patients with successful coronary artery bypass surgery or coronary angioplasty.28
We have shown, for the first time, in the clinical setting that collateral vessels do regress anatomically.29 We re-occluded the culprit lesions in 12 patients who had severe initial stenosis and relatively mild restenosis at an interval of 109 days. We assumed that the extent of ST segment elevation during angioplasty balloon inflation reflected collateral flow in the presence of comparable rate–pressure products. In these patients, the average ST segment elevation was 0.13 mV at initial angioplasty, which is significantly less than the 0.19 mV at repeat angioplasty. Thus, incompletely matured collateral vessels probably are prone to anatomical regression. A limitation of this study must be acknowledged because we used ST segment elevation as a surrogate marker of collateral flow. However, it has recently been confirmed with use of quantitative collateral measurements that the recruitable collateral function is attenuated after successful recanalisation.30
A CRITICISM OF PREVAILING ANGIOGENIC TREATMENT
Despite remarkable advances in medical treatment and revascularisation procedures, a large number of patients with severe coronary artery disease remain symptomatic. Recently, the concept of therapeutic angiogenesis has been proposed by several investigators as an option for the management of these patients.1,2 Therapeutic angiogenesis stimulates the growth of new vessels that collateralise the affected vessel and attenuates myocardial ischaemia in the jeopardised region. It has been shown that administration of angiogenic growth factors, either as a recombinant protein or by gene transfer, can enhance regional myocardial blood flow in animal models. Clinical trials of therapeutic angiogenesis have also indicated not only increases in exercise tolerance associated with reductions in anginal episodes, but also left ventricular functional improvement with evidence of improved perfusion in a number of small open label trials.2,31 However, in two recent double blind, placebo controlled trials with intracoronary recombinant bFGF and intracoronary and subsequent intravenous recombinant human VEGF there was no improvement beyond placebo in exercise tolerance and angina symptoms.32,33
One promising agent for angiogenic treatment, VEGF, is a potent mitogen for endothelial cells. VEGF produced by ischaemic cardiomyocytes stimulates capillary proliferation, and thus contributes to angiogenesis in the ischaemic area. The effects of VEGF are limited to the endothelial cells. Figure 2 is a schematic representation of changes in the resistance of collateral circulation and collateral blood flow before (fig 2A) and after arteriogenesis (fig 2B) and angiogenesis (fig 2C) in patients undergoing these treatment approaches. It is supposed that the resistance in conduit arteries and resistance vessels is equal during reactive hyperaemia and that resistance in resistance vessels increases ninefold in the resting condition.34 Thus, in healthy humans, the coronary flow reserve is approximately 5. In patients with immature collateral vessels, flow reserve is already exhausted at rest in the presence of maximally dilated resistance vessels in the collateral dependent zone (fig 2A). When arteriogenesis of collateral vessels is induced, the resistance of collateral circulation decreases and collateral blood flow increases substantially in the case of reactive hyperaemia (fig 2B). In contrast, angiogenesis in the collateral dependent ischaemic area causes a slight decrease in the vascular resistance in this area. As a result, a definite increase in collateral flow is not expected in this situation (fig 2C).
Figure 2.
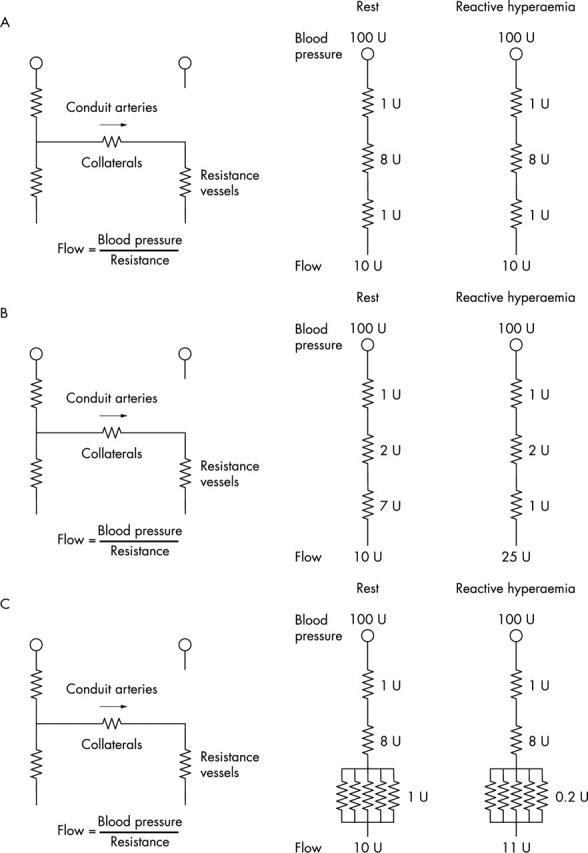
Schematic representation of the mechanisms by which either arteriogenesis or angiogenesis causes an increase in collateral flow reserve. The left coronary artery in the diagram is totally occluded and dependent on collateral channels for perfusion. (A) At baseline, the resistance vessels in the collateral dependent zone are maximally dilated at rest, resulting in no collateral flow reserve. (B) After arteriogenesis, collateral vessel growth causes a 2.5-fold increase in collateral flow reserve. (C) After angiogenesis, the increase in resistance vessels in the collateral dependent zone causes only a 10% increase in collateral flow reserve.
The electrical analogy is based on experimental studies conducted by Scheel et al35,36 with the use of an isolated canine heart preparation with maximal vasodilatation. They showed that arteriogenesis rather than angiogenesis is most likely to link the increased collateral flow. Thus, arteriogenesis of collateral vessels appears to be much more effective than angiogenesis of resistance vessels in the ischaemic zone in terms of collateral flow reserve. Although a slightly increased collateral flow with angiogenesis may lead to arteriogenesis of collaterals by the aforementioned mechanism, treatment intended directly for arteriogenesis of collateral vessels appears to be more reasonable.3–5 In this sense, granulocyte macrophage colony stimulating factor or bFGF may be superior to VEGF because both factors are heavily involved in the process of arteriogenesis.37
FUTURE DIRECTIONS
Collateral vessel recruitment and growth constitute an innate self protective mechanism for severe coronary artery disease. Although strenuous exercise combined with heparin and angiogenic treatment with growth factors is attractive and promising, no treatments to definitely enhance collateral vessel growth are available. In future clinical trials, newly developed realistic methods of assessing collateral growth should be applied.6–8,16,30,37,38 Furthermore, the fundamental concept that an intact supplying artery of collateral circulation is indispensable for sufficient collateral flow should not be forgotten. In this regard, the combination of collateral vessel growth treatment and revascularisation procedures appears to be encouraging.
The rapidly growing alternative strategy for therapeutic angiogenesis is intramyocardial transplantation of cellular components. Kamihata et al39 reported using a swine model in which implantation of bone marrow derived mononuclear cells into the ischaemic area by coronary artery ligation caused improvements in regional blood flow, capillary density, number of angiographically visible collateral vessels, and regional myocardial function. Kawamoto et al40 also showed in a swine ameroid constrictor model that intramyocardial transplantation of autologous endothelial progenitor cells into ischaemic myocardium offered improvements in capillary density in the ischaemic area, Rentrop grade angiographic collateral development, and echocardiographic left ventricular ejection fraction. Clinical application of such a treatment strategy will require further investigation into techniques for harvesting and separating these cells and their ex vivo expansion before intramyocardial transplantation.
Promotion of collateral growth in patients with intractable anginal pain, for whom no effective treatment exists, has many attractive features. If clinicians and researchers working in this field can resolve the remaining practical and mechanistic questions, fruitful progress will be realised in the near future.
Abbreviations
bFGF, basic fibroblast growth factor
VEGF, vascular endothelial growth factor
REFERENCES
- 1.Lee JS, Feldman AM. Gene therapy for therapeutic myocardial angiogenesis: a promising synthesis of two emerging technologies. Nat Med 1998;4:739–42. [DOI] [PubMed] [Google Scholar]
- 2.Simons M, Bonow RO, Chronos NA, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus. Circulation 2000;102:e73. [DOI] [PubMed] [Google Scholar]
- 3.Schaper W, Buschmann I. Arteriogenesis, the good and bad of it. Eur Heart J 1999;20:1297–9. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389–95. [DOI] [PubMed] [Google Scholar]
- 5.Hershey JC, Baskin EP, Glass JD, et al. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res 2001;49:618–25. [DOI] [PubMed] [Google Scholar]
- 6.Pijls NH, van Son JA, Kirkeeide RL, et al. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 1993;87:1354–67. [DOI] [PubMed] [Google Scholar]
- 7.Piek JJ, van Liebergen RA, Koch KT, et al. Pharmacological modulation of the human collateral vascular resistance in acute and chronic coronary occlusion assessed by intracoronary blood flow velocity analysis in an angioplasty model. Circulation 1997;96:106–15. [DOI] [PubMed] [Google Scholar]
- 8.Seiler C, Fleisch M, Billinger M, et al. Simultaneous intracoronary velocity- and pressure-derived assessment of adenosine-induced collateral hemodynamics in patients with one- to two-vessel coronary artery disease. J Am Coll Cardiol 1999;34:1985–94. [DOI] [PubMed] [Google Scholar]
- 9.Sasayama S, Fujita M. Recent insights into coronary collateral circulation. Circulation 1992;85:69–76. [DOI] [PubMed] [Google Scholar]
- 10.Sabia PJ, Powers ER, Ragosta M, et al. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med 1992;327:1825–31. [DOI] [PubMed] [Google Scholar]
- 11.Cribier A, Korsatz L, Koning R, et al. Improved myocardial ischemic response and enhanced collateral circulation with long repetitive coronary occlusion during angioplasty: a prospective study. J Am Coll Cardiol 1992;20:578–86. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Yamanishi K, Inoko M, et al. Preferential dilation of recipient coronary arteries of the collateral circulation by intracoronary administration of nitroglycerin. J Am Coll Cardiol 1994;24:631–5. [DOI] [PubMed] [Google Scholar]
- 13.Ohno A, Fujita M, Miwa K, et al. Importance of coronary collateral circulation for increased treadmill exercise capacity by nitrates in patients with stable effort angina pectoris. Cardiology 1991;78:323–8. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Fujita M, Nakae I, et al. Improvement of exercise capacity by sarpogrelate as a result of augmented collateral circulation in patients with effort angina. J Am Coll Cardiol 1998;32:1982–6. [DOI] [PubMed] [Google Scholar]
- 15.Cohen M, Sherman W, Rentrop KP, et al. Determinants of collateral filling observed during sudden controlled coronary artery occlusion in human subjects. J Am Coll Cardiol 1989;13:297–303. [DOI] [PubMed] [Google Scholar]
- 16.Pohl T, Seiler C, Billinger M, et al. Frequency distribution of collateral flow and factors influencing collateral channel development: functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol 2001;38:1872–8. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Ikemoto M, Kishishita M, et al. Elevated basic fibroblast growth factor in pericardial fluid of patients with unstable angina. Circulation 1996;94:610–3. [DOI] [PubMed] [Google Scholar]
- 18.Nolewajka AJ, Kostuk WJ, Rechnitzer PA, et al. Exercise and human collateralization: an angiographic and scintigraphic assessment. Circulation 1979;60:114–21. [DOI] [PubMed] [Google Scholar]
- 19.Ehsani AA, Heath GW, Hagberg JM, et al. Effects of 12 months of intense exercise training on ischemic ST-segment depression in patients with coronary artery disease. Circulation 1981;64:1116–24. [DOI] [PubMed] [Google Scholar]
- 20.Fujita M. Heparin and angiogenic therapy. Eur Heart J 2000;21:270–4. [DOI] [PubMed] [Google Scholar]
- 21.Tateno S, Terai M, Niwa K, et al. Alleviation of myocardial ischemia after Kawasaki disease by heparin and exercise therapy. Circulation 2001;103:2591–7. [DOI] [PubMed] [Google Scholar]
- 22.Nakae I, Fujita M, Miwa K, et al. Age-dependent impairment of coronary collateral development in humans. Heart Vessels 2000;15:176–80. [DOI] [PubMed] [Google Scholar]
- 23.Abaci A, Oguzhan A, Kahraman S, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation 1999;99:2239–42. [DOI] [PubMed] [Google Scholar]
- 24.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: a potential predictor for the individual capacity to develop collaterals. Circulation 2000;102:185–90. [DOI] [PubMed] [Google Scholar]
- 25.Melidonis A, Tournis S, Kouvaras G, et al. Comparison of coronary collateral circulation in diabetic and nondiabetic patients suffering from coronary artery disease. Clin Cardiol 1999;22:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Belle E, Rivard A, Chen D, et al. Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation 1997;96:2667–74. [DOI] [PubMed] [Google Scholar]
- 27.Yamanishi K, Fujita M, Ohno A, et al. Importance of myocardial ischaemia for recruitment of coronary collateral circulation in dogs. Cardiovasc Res 1990;24:271–7. [DOI] [PubMed] [Google Scholar]
- 28.Sabri MN, DiSciascio G, Cowley MJ, et al. Coronary collateral recruitment: functional significance and relation to rate of vessel closure. Am Heart J 1991;121:876–80. [DOI] [PubMed] [Google Scholar]
- 29.Fujita M, Nakae I, Fudo T, et al. Fate of collateral vessels after successful coronary angioplasty in patients with effort angina. J Am Coll Cardiol 1997;29:544–8. [DOI] [PubMed] [Google Scholar]
- 30.Werner GS, Ferrari M, Betge S, et al. Collateral function in chronic total coronary occlusions is related to regional myocardial function and duration of occlusion. Circulation 2001;104:2784–90. [DOI] [PubMed] [Google Scholar]
- 31.Freedman SB, Isner JM. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol 2001;33:379–93. [DOI] [PubMed] [Google Scholar]
- 32.Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 2002;105:788–93. [DOI] [PubMed] [Google Scholar]
- 33.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 2003;107:1359–65. [DOI] [PubMed] [Google Scholar]
- 34.Winbury MM, Howe BB, Hefner MA. Effect of nitrates and other coronary dilators on large and small coronary arteries: a hypothesis for the mechanism of action of nitrates. J Pharmacol Exp Ther 1969;168:70–95. [PubMed] [Google Scholar]
- 35.Scheel KW, Galindez TA, Cook B, et al. Changes in coronary and collateral flows and adequacy of perfusion in the dog following one and three months of circumflex occlusion. Circ Res 1976;39:654–8. [DOI] [PubMed] [Google Scholar]
- 36.Scheel KW, Wilson JL, Ingram LA, et al. The septal artery and its collaterals in dogs with and without circumflex occlusion. Am J Physiol 1980;238:H504–14. [DOI] [PubMed] [Google Scholar]
- 37.Seiler C, Pohl T, Wustmann K, et al. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation 2001;104:2012–7. [DOI] [PubMed] [Google Scholar]
- 38.Rockstroh J, Brown BG. Coronary collateral size, flow capacity, and growth: estimates from the angiogram in patients with obstructive coronary disease. Circulation 2002;105:168–73. [DOI] [PubMed] [Google Scholar]
- 39.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001;104:1046–52. [DOI] [PubMed] [Google Scholar]
- 40.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 2003;107:461–8. [DOI] [PubMed] [Google Scholar]