Abstract
Objective: To determine the differences in the inflammatory status between diabetic and non-diabetic patients and to evaluate the usefulness of C reactive protein, fibrinogen, and leucocyte count as predictors of death in diabetic patients with unstable coronary disease.
Design: Nested case-control comparisons of the inflammatory status between diabetic and non-diabetic patients. Prospective cohort analysis of C reactive protein concentration, fibrinogen concentration, and leucocyte count as predictors of cardiovascular death in diabetic patients.
Setting: Coronary care unit in Spain.
Participants: 83 diabetic patients with non-ST elevation acute coronary syndrome and 83 sex and aged matched patients selected from 361 non-diabetic patients with non-ST elevation acute coronary syndrome.
Main outcome measures: Plasma concentrations of C reactive protein and fibrinogen, and leucocyte count. Investigators contacted patients to assess clinical events.
Results: Concentrations of C reactive protein and fibrinogen, and leucocyte count on admission were higher in diabetic than in non-diabetic patients (7 mg/l v 5 mg/l, p = 0.020; 3.34 g/l v 2.90 g/l, p = 0.013; and 8.8 × 109/l v 7.8 × 109/l, p = 0.040). Among diabetic patients, these values were also higher in those who died during the 22 month follow up (13 mg/l v 6 mg/l, p = 0.001; 3.95 g/l v 3.05 g/l, p < 0.001; and 11.4 × 109/l v 8.4 × 109/l, p = 0.005). After adjustment for confounding factors, diabetic patients in the highest tertile of C reactive protein had a hazard ratio for cardiovascular death of 4.51 (95% confidence interval (CI) 1.62 to 12.55). Similar hazard ratios were for fibrinogen 3.74 (95% CI 1.32 to 10.62) and for leucocyte count 3.64 (95% CI 1.37 to 9.68).
Conclusions: Inflammation appears more evident in diabetic than in non-diabetic patients with acute coronary syndrome. C reactive protein concentration, fibrinogen concentration, and leucocyte count constitute independent predictors of cardiovascular death in diabetics with unstable coronary disease.
Keywords: inflammation, diabetes mellitus, C reactive protein, fibrinogen, leucocyte count, acute coronary syndrome
Diabetes mellitus is widely recognised as being perhaps the most significant risk factor for the development of acute coronary syndromes.1–2 Furthermore, when patients with diabetes develop clinical events, their prognosis is worse than that for non-diabetics.3
The basis for the excess risk of cardiovascular disease among diabetic patients has not been completely determined. Firstly, there is a high prevalence of conventional risk factors such as dyslipidaemia (high triglycerides, low high density lipoprotein, and small dense low density lipoprotein particles), hypertension, and obesity.4–6 Secondly, diabetic patients are at increased risk for thrombosis formation as a consequence of increased platelet reactivity, increased concentrations and activity of coagulation factors, and decreased activity of antithrombotic factors and fibrinolytic system capacity resulting from overexpression of plasminogen activator inhibitor type 1.7–10 Thirdly, insulin resistance, hyperinsulinaemia, hyperglycaemia, and advanced glycation end products also affect arterial wall physiology.11–14 All these mechanisms favour systemic and coronary inflammation and accelerated progression and precipitation of atherothrombosis.15,16 In fact, in diabetic patients with unstable angina coronary plaques have a higher incidence of plaque ulceration and intracoronary thrombus formation than in non-diabetics patients. Diabetic plaques usually have a greater lipid core burden and a richer inflammatory component and are more commonly complicated by overlying thrombosis.17 In addition, inflammation is an important pathogenetic determinant of type 2 diabetes. C reactive protein concentration and leucocyte count have been shown to be independent predictors of the development of type 2 diabetes.18,19 Anti-inflammatory agents, such as statins,20 peroxisome proliferator activated receptor agonists,21 and angiotensin converting enzyme inhibitors may also delay the onset of diabetes in high risk patients.22
Recently, systemic blood markers of inflammation have emerged as powerful predictors of coronary events in patients with unstable angina and after myocardial infarction.23–29 However, very limited information is available concerning the concentrations of C reactive protein and fibrinogen and of leucocyte count in diabetic patients with unstable coronary artery disease. Thus, the present study aimed at determining the differences in the inflammatory status between non-diabetic and diabetic patients with non-ST elevation acute coronary syndrome, and evaluating and comparing the usefulness of C reactive protein concentration, fibrinogen concentration, and leucocyte count as predictors of long term mortality in diabetic patients.
METHODS
Patient population
A total of 83 of 119 consecutive patients with type 2 diabetes admitted to our coronary care unit with a diagnosis of non-Q wave myocardial infarction or Braunwald class IIIB unstable angina from October 1998 to June 2000 were included in the study. Patients with ST elevation at admission (four patients), left bundle branch block (two patients), new Q waves of more than 0.04 seconds in at least two leads (two patients), type 1 diabetes (one patient), malignancy or inflammatory disease (six patients), surgery or major trauma in the previous two months (two patients), valvar heart disease (six patients), patients with previous myocardial infarction and ejection fraction < 40% (four patients), time from onset of symptoms to admission to the coronary care unit > 12 hours (five patients), and creatinine ⩾ 176 µmol/l were excluded from the study (four patients). To compare the differences in the inflammatory status between diabetic and non-diabetic patients, the study group was sex and age matched to 83 of 361 non-diabetic patients admitted to our coronary care unit with the same diagnosis and in the same period of time. All patients were initially treated medically and underwent coronary angiography and revascularisation when refractory or incapacitating angina and signs of severe ischaemia during exercise testing were present. Patients were followed up in a prospective manner. After patients were discharged, investigators contacted patients to assess clinical events. Clinical follow up was available for all patients (100%). Median follow up of diabetic patients was 22 months.
Laboratory assays and study protocol
Venous blood samples were obtained from all patients at the time of admission to the coronary care unit. C reactive protein concentrations were determined by turbidimetry with a commercially available kit (Hitachi model 717, Boehringer Mannheim, Mannheim, Germany). The lower detection limit was 1.3 mg/l. The interassay coefficient of variation was 6% and the intra-assay coefficient was 1.3%. A comparison of the C reactive protein determination with a nephelometric method gave a correlation coefficient of 0.991. Plasma concentrations of fibrinogen were measured by use of the Clauss method. Troponin I concentrations were measured by a paramagnetic particle chemiluminescent immunoenzymatic assay. Diabetes mellitus was defined as a fasting serum glucose concentration of 7.0 mmol/l (126 mg/dl) or more, a non-fasting glucose concentration of 11.1 mmol/l (200 mg/dl) or more, the participant’s report of a physician’s diagnosis of diabetes, or current use of diabetes medication.30
Statistical analysis
C reactive protein and fibrinogen concentrations and leucocyte count are presented as median and interquartile range. Non-parametric tests were used to compare C reactive protein and fibrinogen concentrations and leucocyte counts between groups (Mann-Whitney U test) and to determine correlations (Spearman’s p test). Discontinuous variables were tested by a contingency χ2 test. Diabetic patients were divided into tertiles on the basis of their C reactive protein and fibrinogen concentrations and leucocyte counts at admission. Diabetic patients in the highest tertile were compared with those with concentrations in the two lower tertiles. By forward stepwise logistic regression analysis, we calculated the relative odds ratios and 95% confidence intervals (CIs) for in-hospital cardiac death. We used Cox regression analyses with forward stepwise selection to calculate the adjusted hazard ratios and 95% CIs for death from cardiovascular causes for the total follow up period. Logistic and Cox regression analyses considered age ⩾ 65 years, sex, body mass index, current smoking, presence or absence of hypertension, previous coronary artery disease, presence or absence of a history of hyperlipidaemia, two or more anginal events in the previous 24 hours, ST segment depression ⩾ 0.5 mm at admission, troponin I >0.2 ng/ml on admission, diabetes treatment status, and the presence or absence of prior treatment with aspirin and statins. C reactive protein and fibrinogen concentrations and leucocyte count were thus entered as dichotomised variables, upper tertile versus lower tertile. For comparison with recent studies,31 we evaluated the additive effect of C reactive protein and fibrinogen concentrations and leucocyte count in combination. We categorised diabetic patients on the basis of the number of increased inflammatory markers on admission: none, one, two, or all three. For the present analysis we used a cut off value of 10 mg/l for C reactive protein,32 10 000 cells/dl for leucocyte count,28 and > 4.00 g/l for fibrinogen.29
Finally, event-free survival was analysed by the Kaplan-Meier method and the log rank test was used for to compare curves. A two sided probability value of p < 0.05 was considered significant.
RESULTS
Table 1 shows the baseline clinical characteristics of non-diabetic and diabetic patients. There were no differences in baseline characteristics between groups except for a higher smoking history in non-diabetic patients (43 (51.8%) v 27 (32.5%), p = 0.01). Almost one third of the patients in both groups had had a previous myocardial infarction, were taking aspirin at admission, and had higher concentrations of troponin I. Approximately 46% of non-diabetic and 55% of diabetic patients had ST segment depression ⩾ 0.5 mm on admission to the hospital. The mean time from the onset of chest pain to admission in the coronary care unit for diabetic patients was 4.6 (3.3) hours, similar to that for non-diabetic patients. The median C reactive protein and fibrinogen concentrations and the leucocyte counts were significantly higher at admission in the diabetic than in the non-diabetic patients (table 1).
Table 1.
Clinical data of non-diabetic and diabetic patients
| Non-diabetic (n = 83) | Diabetic (n = 83) | p Value | |
| Median age (years) | 71 | 71 | 1.000 |
| Female sex | 26 (31.3%) | 26 (31.3%) | 1.000 |
| Hypertension | 36 (43.4%) | 39 (47.0%) | 0.640 |
| Smoking history | 43 (51.8%) | 27 (32.5%) | 0.012 |
| Hypercholesterolaemia | 46 (55.4%) | 47 (56.6%) | 0.876 |
| Body mass index ⩾25 kg/m2 | 49 (59.0%) | 52 (62.7%) | 0.633 |
| Stable angina >3 months before admission | 22 (26.5%) | 31 (37.3%) | 0.134 |
| Previous myocardial infarction | 22 (26.5%) | 24 (28.9%) | 0.729 |
| Aspirin at admission | 31 (37.3%) | 26 (31.3%) | 0.414 |
| Statins at admission | 13 (15.7%) | 10 (12.0%) | 0.500 |
| ⩾2 anginal episodes in previous 24 hours | 37 (44.6%) | 28 (33.7%) | 0.152 |
| ⩾ST segment depression ⩾0.5 mm at admission | 38 (45.8%) | 46 (55.4%) | 0.214 |
| Coronary angiography during the study | 39 (47.0%) | 34 (41.0%) | 0.434 |
| One vessel disease | 13 (66.7%) | 8 (23.5%) | 0.356 |
| Multivessel disease | 26 (33.3%) | 26 (76.5%) | 0.356 |
| Troponin I ⩾0.2 ng/ml at admission* | 32 (38.6%) | 29 (34.9%) | 0.629 |
| Mean (SD) time from onset of chest pain to admission (hours) | 4.7 (3.0) | 4.6 (3.3) | 0.785 |
| Median C reactive protein (mg/l) | 5 (2, 8) | 7 (3, 11) | 0.020 |
| Median fibrinogen (g/l) | 2.90 (2.44, 3.33) | 3.34 (2.62, 3.92) | 0.013 |
| Median leucocyte (×109/l) | 7.8 (6.5, 10.1) | 8.8 (6.9, 11.3) | 0.040 |
*Twice the upper limit of the authors’ laboratory.
The median duration of follow up of diabetic patients was 22 months. Nine (10.8%) diabetic patients died during hospitalisation and 18 (21.7%) during the entire follow up time (table 2). Compared with those who survived during the follow up, diabetic patients who died were older and a higher percentage were women. A larger percentage also were not taking statins and had ST segment depression at admission (table 3).
Table 2.
Cause of death
| Cause of death | Number |
| Total cardiovascular mortality | 18 (21.7%) |
| In-hospital mortality | 9 (10.8%) |
| Congestive heart failure | 7 |
| Acute, fulminating pulmonary oedema within 24 hours of admission (severe MR) | 2 |
| Acute, fulminating pulmonary oedema > 24 hours after admission (severe MR) | 1 |
| Hypotension and cardiogenic shock | 2 |
| Cardiorrhexis | 2 |
| Arrhythmias | 1 |
| Reinfarction | 1 |
| Mortality after discharge | 9 (10.8%) |
| Congestive heart failure | 4 |
| Reinfarction | 2 |
| Sudden death | 2 |
| Aortic aneurysm rupture | 1 |
MR, mitral regurgitation.
Cardiorrhexis was defined as sudden death with electromechanical dissociation.
Table 3.
Clinical data of surviving and non-surviving diabetic patients
| Surviving (n = 65) | Non-surviving (n = 18) | p Value | |
| Median age (years) | 69 | 76 | 0.001 |
| Female sex | 16 (24.6%) | 10 (55.6%) | 0.012 |
| Hypertension | 31 (47.7%) | 8 (44.4%) | 0.807 |
| Smoking history | 24 (36.9%) | 3 (16.7%) | 0.105 |
| Hypercholesterolaemia | 32 (49.2%) | 11 (61.1%) | 0.372 |
| Body mass index ⩾ 25 kg/m2 | 43 (66.2%) | 9 (50.0%) | 0.210 |
| Stable angina > 3 months before admission | 22 (33.8%) | 9 (50.0%) | 0.210 |
| Previous myocardial infarction | 18 (27.7%) | 6 (33.3%) | 0.640 |
| Aspirin at admission | 20 (30.8%) | 6 (33.3%) | 0.640 |
| Statins at admission | 13 (20.0%) | 0 | 0.039 |
| ⩾2 anginal episodes in previous 24 hours | 22 (33.8%) | 6 (33.3%) | 0.968 |
| ST segment depression ⩾0.5 mm at admission | 32 (49.2%) | 14 (77.8%) | 0.031 |
| Troponin I ⩾0.2 ng/ml at admission* | 20 (30.8%) | 9 (50.0%) | 0.130 |
| Median C reactive protein (mg/l) | 6 (3, 9) | 13 (7, 39) | 0.001 |
| Median fibrinogen (g/l) | 3.05 (2.51, 3.80) | 3.95 (3.55, 4.65) | <0.001 |
| Median leucocyte count (109/l) | 8.4 (6.7, 10.3) | 11.4 (8.7, 12.9) | 0.005 |
*Twice the upper limit of the authors’ laboratory.
The median C reactive protein and fibrinogen concentrations and the leucocyte count were higher in diabetic patients who died than in those who survived during hospitalisation (18 mg/l v 6 mg/l, p = 0.01; 3.89 g/l v 3.18 g/l, p = 0.004; and 11.7 ×109/l v 8.6 ×109/l, p = 0.054) and during the entire follow up (13 mg/l v 6 mg/l, p = 0.001; 3.95 g/l v 3.05 g/l, p < 0.001; and 11.4 ×109/l v 8.4 ×109/l, p = 0.005).
The correlation coefficient between C reactive protein concentration and fibrinogen concentration was 0.43 (p < 0.001), between C reactive protein concentration and leucocyte count was 0.18 (p = 0.105), and between fibrinogen concentration and leucocyte count was 0.27 (p = 0.015). The coefficient correlations between troponin I and each of C reactive protein concentration, fibrinogen concentration, and leucocyte count at admission were weak and not significant (0.11, −0.01, and 0.07, respectively).
Coronary angiography was performed in 34 diabetic patients (41%); 13 (15.7%) before discharge and 21 (25.3%) during the follow up. No patients had normal coronary arteries (coronary stenosis < 50%). Eight, eight, and 18 patients had one, two, or three vessel coronary disease, respectively. Two (5.9%) of the 34 diabetic patients had significant left main artery disease. The Kruskal-Wallis one way analysis of variance indicated that there was no relation between the number of diseased coronary arteries and either C reactive protein concentration (p = 0.453), fibrinogen concentration (p = 0.126), or leucocyte count (p = 0.548). No association was found between concentrations of the three inflammatory markers or with lesion morphology.
Table 4 shows the incidence of clinical events according to tertiles in which diabetic patients were divided on the basis of their C reactive protein and fibrinogen concentration and their leucocyte counts at admission. The Kaplan-Meier analysis showed that patients with the highest C reactive protein and fibrinogen concentrations and leucocyte count had a significantly higher probability of death from cardiac causes during the entire follow up period than patients with lower values (fig 1).
Table 4.
Incidence of cardiovascular death and events during hospitalisation and follow up
| Tertile 1 | Tertile 2 | Tertile 3 | p Value | ||
| C reactive protein | Range (mg/l) | <5 | 5–8 | >8 | |
| Number | 28 | 26 | 29 | ||
| Follow up mortality | 1 (3.6%) | 5 (19.2%) | 12 (41.4%) | 0.002 | |
| In-hospital mortality | 1 (3.6%) | 1 (3.8%) | 7 (24.1%) | 0.017 | |
| Follow up revascularisation | 5 (17.4%) | 10 (38.5%) | 6 (20.7%) | 0.171 | |
| In-hospital revascularisation | 3 (10.7%) | 2 (7.7%) | 2 (6.9%) | 0.863 | |
| Follow up reinfarction | 3 (10.%7) | 3 (11.5%) | 5 (17.2%) | 0.733 | |
| Fibrinogen | Range (g/l) | <2.81 | 2.81–2.78 | >2.78 | |
| Number | 28 | 27 | 28 | ||
| Follow up mortality | 1 (3.6%) | 6 (22.2%) | 11 (39.3%) | 0.005 | |
| In-hospital mortality | 0 | 3 (11.1%) | 6 (21.4%) | 0.036 | |
| Follow up revascularisation | 8 (28.6%) | 9 (33.3%) | 4 (14.3%) | 0.096 | |
| In-hospital revascularisation | 5 (17.9%) | 1 (3.7%) | 1 (3.6%) | 0.088 | |
| Follow up reinfarction | 5 (17.9%) | 1 (3.7%) | 5 (17.9%) | 0.205 | |
| Leucocyte count | Range (×109/l) | <7.5 | 7.6–10.2 | >10.3 | |
| Number | 29 | 26 | 28 | ||
| Follow up mortality | 4 (13.8%) | 3 (11.5%) | 11 (39.3%) | 0.0214 | |
| In-hospital mortality | 2 (6.9%) | 0 | 7 (25.0%) | 0.009 | |
| Follow up revascularisation | 10 (34.5%) | 7 (26.9%) | 4 (14.3%) | 0.807 | |
| In-hospital revascularisation | 5 (17.2%) | 2 (7.7%) | 0 | 0.064 | |
| Follow up reinfarction | 4 (13.8%) | 2 (7.7%) | 5 (17.9%) | 0.543 | |
Figure 1.
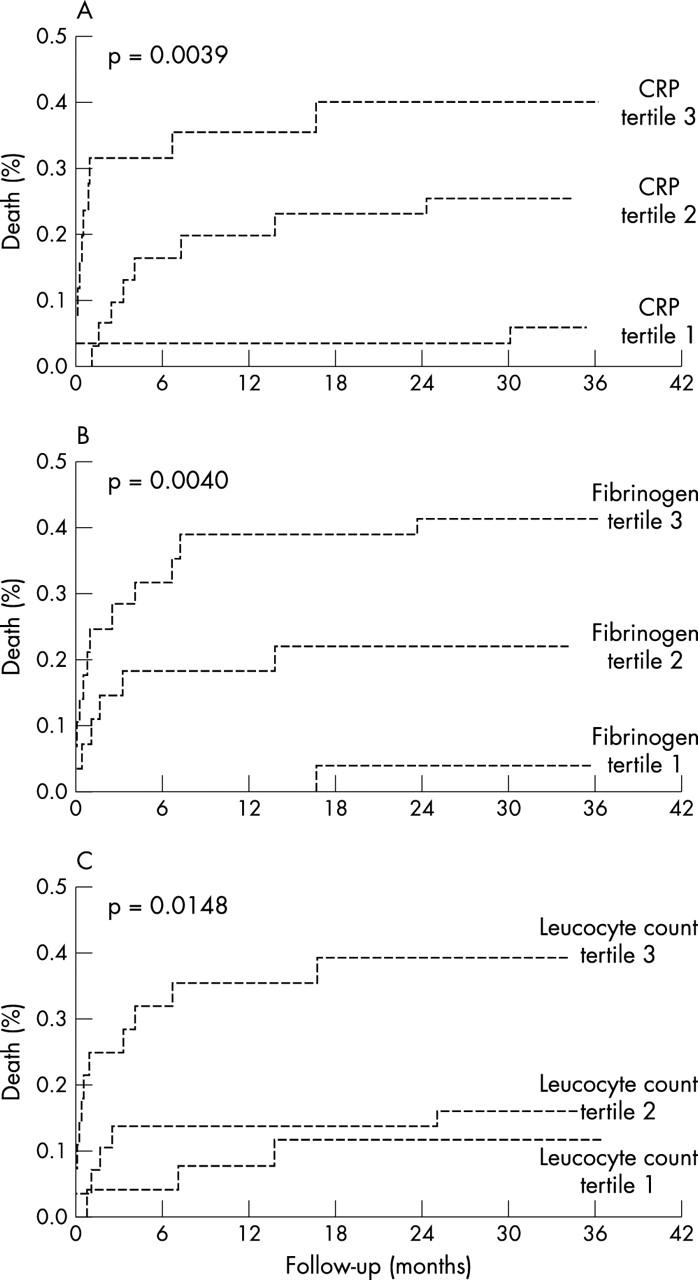
Kaplan-Meier survival estimates for diabetic patients divided into tertiles according to (A) C reactive protein concentrations, (B) fibrinogen concentrations, and (C) leucocyte count on admission.
Multivariable analysis of the relations between clinical data, myocardial damage markers, findings on the ECG, serum inflammatory markers determined, and the risk of cardiovascular death showed that two variables were significant for in-hospital mortality (C reactive protein concentrations and leucocyte count at admission) and four variables for the entire follow up period (C reactive protein, fibrinogen, leucocyte count, and female sex). The adjusted odds ratios for in-hospital mortality were 9.19 (95% CI 1.60 to 52.74; p = 0.013) for diabetic patients with C reactive protein in the top third of measurements and for leucocyte count 9.79 (95% CI 1.71 to 56.04; p = 0.010). The hazard ratios for death during the entire follow up were 4.51 (95% CI 1.62 to 12.55; p = 0.004) for diabetic patients in the highest tertile of C reactive protein, 3.74 (95% CI 1.32 to 10.62; p = 0.013) for patients in the highest tertile of fibrinogen, and 3.64 (95% CI 1.37 to 9.68; p = 0.01) for leucocyte count. Finally, the hazard ratio for female sex diabetic patients was 3.45 (95% CI 1.30 to 9.09; p = 0.013).
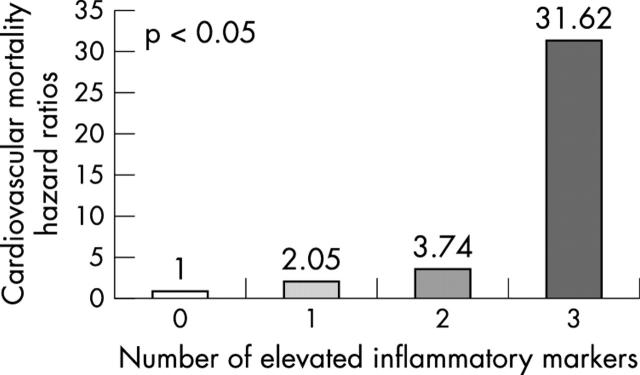
Categorising patients on the basis of the number of increased inflammatory markers on admission, 43.5% had no increased markers, 37.3% had one, 12% had two, and 7.2% had increases in all three. Fig 2 shows adjusted hazard ratios for cardiovascular death in diabetic patients stratified by the number of increased inflammatory markers.
Figure 2.
Cardiovascular death hazard ratios in diabetic patients based on the number of increased inflammatory markers.
DISCUSSION
Coronary tissue from diabetic patients with acute coronary syndrome has more lipid rich atheroma, thrombosis, and macrophage cell infiltration than tissue from patients without diabetes. This higher incidence of inflammatory cell infiltration in coronary tissue from diabetic patients suggests not only that inflammation may play an important part in the pathophysiology of acute coronary syndrome but also that a more proinflammatory state is present in diabetic than in non-diabetic patients. The observation of increased concentrations of C reactive protein and other acute phase reactants in diabetes is not new; reports date back to the 1980s. Among > 16 000 patients in the NHANES III (national health and nutrition examination survey III), C reactive protein was shown to be higher in patients with diabetes or glucose intolerance than in control participants.33,34 In consideration of the overlap between inflammatory status and diabetes, some authors have also looked at the association between these two conditions among patients with acute coronary syndrome. In the FRISC (Fragmin during instability in coronary artery disease) study diabetes and C reactive protein concentrations on admission were shown to be independent predictors of long term outcome.23 Interestingly, in some series the presence of diabetes has been associated, independent of the prognosis, with increased C reactive protein concentrations,24,25 whereas in other studies the two parameters appeared to be independent.26 In our study diabetic patients with acute coronary disease had an enhanced underlying inflammatory reaction, as their C reactive protein concentrations, fibrinogen concentrations, and leucocyte counts at admission were higher than in non-diabetics. Myocardial necrosis may account for some of the increase found in C reactive protein, fibrinogen, and leucocytes. However, troponin I concentrations were similar at admission in both groups and the correlations between troponin I and C reactive protein, fibrinogen, or leucocytes in diabetic patients were also weak, as previous studies of non-diabetic patients have shown.23,27
Diabetic patients who suffer a myocardial infarction have a high mortality both acutely and on long term follow up. This increased in-hospital mortality among diabetic patients with acute coronary syndromes is attributable predominantly to a higher increased incidence of reinfarction and recurrent ischaemia, and mainly to congestive heart failure.35–37 Furthermore, cardiogenic shock is more common and more severe in diabetic patients than would be expected from the size of the index infarction.38 Despite the size of the infarction, pre-existing diastolic dysfunction and reduced global and regional left ventricular ejection fraction of the non-infarcted myocardium39,40 are major culprits of the congestive symptoms. Late mortality is principally related to recurrent myocardial infarction and the development of new congestive heart failure.36,38 We also found that congestive heart failure was the main contributing factor not only of in-hospital mortality (78%) but also of late mortality (44%). Our finding of enhanced inflammatory response may explain the higher mortality found in diabetic patients after unstable coronary disease and may help future treatments—glycoprotein IIb/IIIa inhibitor, aspirin, and some cholesterol lowering drugs exert a beneficial influence on inflammation markers and clinical outcomes.41
A variety of clinical studies have been carried out to investigate different markers of inflammation as predictors of coronary events in patients with unstable angina and after myocardial infarction. Although several of them have been shown to predict risk independently of conventional risk factors, the question remains which marker should be preferred in the routine clinical setting. To our knowledge very limited information is available concerning diabetic patients, although one of the goals of this study was to evaluate and compare the usefulness of C reactive protein concentration, fibrinogen concentration, and leucocyte count as predictors of death in diabetic patients. The association between these serum inflammatory markers in diabetic patients and the risk of death was independent of the presence or absence of other classical risk factors: troponin I concentration, electrocardiographic changes, prior treatment with aspirin or statins, and other known predictors of the risk of death. Leucocyte count and C reactive protein concentration were strong and similar predictors of in-hospital mortality in our study, and both were superior to fibrinogen concentration. C reactive protein developed as the most significant predicting factor of cardiovascular death in the entire follow up. As leucocyte count is widely assessed in patients admitted with acute coronary syndrome we must not forget that it may provide clinicians with a simple tool for risk stratification. In our opinion, our data suggest that at admission C reactive protein concentration should be routinely determined in the initial evaluation of diabetic patients admitted with acute coronary syndrome. Simultaneous assessment of C reactive protein concentration, fibrinogen concentration, and leucocyte count also provides prognostic information and may have practical clinical applications in risk stratification and targeting of treatment of diabetic patients with unstable coronary disease. Moreover, categorising diabetic patients based on the number of increased inflammatory markers may counteract the intraindividual variability in the estimation of these inflammatory markers.42
Conclusions
Our study showed enhanced underlying inflammatory reaction in diabetic patients with acute coronary disease as shown by C reactive protein concentration, fibrinogen concentration, and leucocyte count on admission that were higher than those of non-diabetic patients. More interestingly, these markers of inflammation are independent predictors of cardiovascular death in diabetics with unstable non-ST elevation acute coronary syndrome.
Acknowledgments
Thanks to Dr Carla Criado, Dr Jesus Herrero, and Dr Pablo Prieto for their participation in the collection of data.
Abbreviations
CI, confidence interval
FRISC, Fragmin during instability in coronary artery disease
NHANES III, national health and nutrition examination survey III
REFERENCES
- 1.Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care 1993;16:434–44. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz CJ, Valente AJ, Sprague EA, et al. Pathogenesis of the atherosclerotic lesion. Implication for diabetes mellitus. Diabetes Care 1992;15:1156–67. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RD, Cupples A, Schaefer EJ, et al. Lipoproteins, apolipoproteins, and low-density lipoprotein size among diabetics in the Framingham offspring study. Metabolism 1996;45:1267–72. [DOI] [PubMed] [Google Scholar]
- 5.Stamler J, Vaccaro O, Neaton JD, et al. The multiple risk factor intervention trial group: diabetes, other risk factors and 12-year cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care 1993;16:434–44. [DOI] [PubMed] [Google Scholar]
- 6.Sowers JR. Obesity and cardiovascular disease. Clin Chem 1998;44:1821–5. [PubMed] [Google Scholar]
- 7.Tschoepe D, Roesen P, Schwippert B, et al. Platelets in diabetes: the role in the hemostatic regulation in atherosclerosis. Semin Thromb Hemost 1993;19:122–8. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB, D’Agostino RB, Wilson PW, et al. Diabetes, fibrinogen, and the risk of cardiovascular disease: the Framingham experience. Am Heart J 1990;120:672–6. [DOI] [PubMed] [Google Scholar]
- 9.Schneider DJ, Nordt TK, Sobel BE. Attenuated fibrinolysis and accelerated atherogenesis in type II diabetes patients. Diabetes 1993;42:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Nordt TK, Schneider DJ, Sobel BE. Augmentation of the synthesis of plasminogen activator inhibitor type-1 by precursor of insulin. A potential risk factor for vascular disease. Circulation 1994;89:321–30. [DOI] [PubMed] [Google Scholar]
- 11.Ferranini E, Haffner SM, Mitchell BM, et al. Hyperinslulinaemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia 1991;34:416–22. [DOI] [PubMed] [Google Scholar]
- 12.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on and old paradigm. Diabetes 1999;48:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities-the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996;334:374–82. [DOI] [PubMed] [Google Scholar]
- 14.Bucala R, Makita Z, Koschinsky T, et al. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci USA 1993;90:6434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J 2002;23:831–4. [DOI] [PubMed] [Google Scholar]
- 16.Biondi-Zoccai GGL, Abbate A, Liuzzo G, et al. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003;41:1071–7. [DOI] [PubMed] [Google Scholar]
- 17.Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000;102:2180–4. [DOI] [PubMed] [Google Scholar]
- 18.Freeman DJ, Norrie J, Caslake MJ, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the west of Scotland coronary prevention study. Diabetes 2002;51:1596–600. [DOI] [PubMed] [Google Scholar]
- 19.Ford ES. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of U.S. adults. Am J Epidemiol 2002;155:57–64. [DOI] [PubMed] [Google Scholar]
- 20.Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the west of Scotland coronary prevention study. Circulation 2001;103:357–62. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2796–803. [DOI] [PubMed] [Google Scholar]
- 22.Anon. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcome Prevention Evaluation Study Investigators. N Engl J Med 2000;342:145–53. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl B, Toss H, Siegbahn A, for the FRISC Study Group, et al. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC study group. Fragmin during instability in coronary artery disease. N Engl J Med 2000;343:1139–47. [DOI] [PubMed] [Google Scholar]
- 24.Ferreiros ER, Boissonnet CP, Pizarro R, et al. Independent prognostic value of elevated C-reactive protein in unstable angina. Circulation 1999;100:1958–63. [DOI] [PubMed] [Google Scholar]
- 25.Morrow DA, Rifai N, Antman EM, et al. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: A TIMI 11A substudy. J Am Coll Cardiol 1998;31:1460–5. [DOI] [PubMed] [Google Scholar]
- 26.Heeschen C, Hamm CW, Bruemmer J, et al. Predictive value of C-reactive protein and troponin T in patients with unstable angina: a comparative analysis. CAPTURE Investigators. Chimeric c7E3 antiplatelet therapy in unstable angina refractory to standard treatment trial. J Am Coll Cardiol 2000;35:1535–42. [DOI] [PubMed] [Google Scholar]
- 27.Toss H, Lindahl B, Siegbahn A, et al. Prognostic influence of increased fibrinogen and C-reactive protein levels in unstable coronary artery disease. Circulation 1997;96:4204–10. [DOI] [PubMed] [Google Scholar]
- 28.Biasucci LM, Liuzzo G, Grillo RL, et al. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation 1999;99:855–60. [DOI] [PubMed] [Google Scholar]
- 29.Cannon CP, McCabe CH, Wilcox RG, et al. Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. Am J Cardiol 2001;87:636–9. [DOI] [PubMed] [Google Scholar]
- 30.Kahn R, for the American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–96. [DOI] [PubMed] [Google Scholar]
- 31.Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes. Simultaneous assessment of troponin I, C-Reactive protein, and B-Type natriuretic peptide. Circulation 2002;105:1760–3. [DOI] [PubMed] [Google Scholar]
- 32.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 33.McMillan DE. Increased levels of acute phase serum proteins in diabetes. Metabolism 1989;38:1042–6. [DOI] [PubMed] [Google Scholar]
- 34.Ford ES. Body mass index, diabetes, and C-reactive protein among US adults. Diabetes Care 199;22:1971–7. [DOI] [PubMed] [Google Scholar]
- 35.Malmberg K, Ryden L. Myocardial infarction in patients with diabetes mellitus. Eur Heart J 1988;9:259–64. [DOI] [PubMed] [Google Scholar]
- 36.Stone PH, Muller JE, Hartwell T, et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol 1989;14:49–57. [DOI] [PubMed] [Google Scholar]
- 37.Ulvenstam G, Aberg A, Bergstrand R, et al. Long-term prognosis after myocardial infarction in men with diabetes. Diabetes 1985;34:787–92. [DOI] [PubMed] [Google Scholar]
- 38.Jaffe AS, Spadaro JJ, Schechtman K, et al. Increased congestive hear failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J 1988;108:31–7. [DOI] [PubMed] [Google Scholar]
- 39.Iwasaka T, Takahashi N, Nakamura S, et al. Residual left ventricular pump function after acute myocardial infarction in NIDDM patients. Diabetes Care 1992;15:1522–6. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi N, Iwasaka T, Sugiura T, et al. Left ventricular regional function after acute anterior myocardial infarction in diabetic patients. Diabetes Care 1989;12:630–5. [DOI] [PubMed] [Google Scholar]
- 41.Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomised trial and cohort study. JAMA 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 42.Clark GH, Fraser CG. Biological variation of acute phase proteins. Ann Clin Biochem 1993;30:373–6. [DOI] [PubMed] [Google Scholar]