Abstract
Objective: To evaluate N-terminal pro-brain natriuretic peptide (NT-proBNP) as a diagnostic and prognostic marker for systolic heart failure in the general population.
Design: Study participants, randomly selected to be representative of the background population, filled in a heart failure questionnaire and underwent pulse and blood pressure measurements, electrocardiography, echocardiography, and blood sampling and were followed up for a median (range) period of 805 (60−1171) days.
Setting: Participants were recruited from four randomly selected general practitioners and were examined in a Copenhagen university hospital.
Patients: 382 women and 290 men in four age groups (50−59 (n = 174); 60−69 (n = 204); 70−79 (n = 174); ⩾ 80 years (n = 120)).
Main outcome measures: Value of NT-proBNP in evaluating patients with symptoms of heart failure and impaired left ventricular (LV) systolic function; prognostic value of NT-proBNP for mortality and hospital admissions.
Results: In 38 (5.6%) participants LV ejection fraction (LVEF) was ⩽ 40%. NT-proBNP identified patients with symptoms of heart failure and LVEF ⩽ 40% with a sensitivity of 0.92, a specificity of 0.86, positive and negative predictive values of 0.11 and 1.00, and area under the curve of 0.94. NT-proBNP was the strongest independent predictor of mortality (hazard ratio (HR) = 5.70, p < 0.0001), hospital admissions for heart failure (HR = 13.83, p < 0.0001), and other cardiac admissions (HR = 3.69, p < 0.0001). Mortality (26 v 6, p = 0.0003), heart failure admissions (18 v 2, p = 0.0002), and admissions for other cardiac causes (44 v 13, p < 0.0001) were significantly higher in patients with NT-proBNP above the study median (32.5 pmol/l).
Conclusions: Measurement of NT-proBNP may be useful as a screening tool for systolic heart failure in the general population.
Keywords: N-terminal pro-brain natriuretic peptide, diagnostic techniques, ejection fraction, heart failure, population study, prognosis
Until recently, heart failure has been a much neglected condition, probably resulting from a combination of lack of good epidemiological data, lack of simple diagnostic techniques, and lack of effective treatment. However, within the past few years, a large amount of epidemiological data has been presented,1–4 indicating that heart failure is a common, underdiagnosed, and serious condition with a dismal prognosis, comparable with several forms of cancer.5 In the same period, effective treatments for heart failure have become available, including angiotensin converting enzyme inhibitors,6 β blockers,7 and spironolactone,8 creating a need for effective screening tools for the disease. However, diagnosis of heart failure still relies mainly on clinical evaluation and an echocardiographic measure of left ventricular (LV) systolic function.9 In an attempt to facilitate and improve diagnosis, biochemical substances have been suggested as alternative markers for the disease. Efforts have focused on the natriuretic peptides, among which brain natriuretic peptide (BNP)10,11 and its amino terminal portion N-terminal pro-brain natriuretic peptide (NT-proBNP),12 which may hold advantages because of its greater stability,13 have proved especially promising. Concentrations of BNP and NT-proBNP are related to LV filling pressures14 and wall stress.15 In previous population based studies by McDonagh and colleagues, BNP has been identified as a promising prognostic16 and diagnostic marker for impaired LV systolic function.17 However, community based diagnostic or prognostic heart failure data on NT-proBNP are completely lacking.
The purpose of our study was to evaluate NT-proBNP as a diagnostic marker for systolic heart failure and as a prognostic marker for mortality and cardiac morbidity in a large age controlled sample of the general population.
METHODS
Patients
The study population was recruited from four Copenhagen general practitioners located in the same urban area. The only inclusion criterion was age between 50 and 90 years. Exclusion criteria were inability to cooperate (for example, for mental reasons such as dementia), residency in nursing homes, and lack of response to two written invitations.
Study design
To have a sufficient number of elderly participants, the study sample was stratified to have at least 120 people in each decade. An invitation to participate in the study was sent to all people between 50 and 90 years of age assigned to the first two general practitioners. From the third practice all those aged 60–90 years and from the last practice all those aged 80–90 years were invited. Seventy per cent of those invited participated in the study. The attendance was higher among younger invitees, approximately 75%, and was about 50% in the oldest decade. The study was designed to be representative of the background population in Copenhagen by compensating for the lower response rate in the older age groups through oversampling in these groups. Participants from general practitioners 1 and 2 entered the study in the autumn of 1997 and were submitted to a questionnaire, an echocardiogram, and a blood pressure measurement. They were recalled by the autumn of 1998 (n = 424), when an ECG was recorded and their blood sampled. Patients from general practitioner 3 (n = 207) and general practitioner 4 (n = 41) were submitted to all examinations in the autumn and winter 1998/99. Thus, all patients in the study were effectively examined once. The local ethics committee approved the study and the patients gave written informed consent.
Heart failure questionnaire
All subjects filled in a heart failure questionnaire on medical history, symptoms, drug history, and alcohol and tobacco consumption. Symptoms of heart failure were recorded from questions on ankle swelling and breathlessness (self reported). The degree of breathlessness was recorded from the questionnaire according to the World Health Organization classification (grade 1, no dyspnoea; grade 2, dyspnoea on vacuum cleaning or climbing stairs to the second floor; grade 3, dyspnoea when walking on an even road; grade 4, dyspnoea with minimum exertion; grade 5, orthopnoea; grade 6, dyspnoea at rest).17 Self reported medical history was recorded from questions on hospital admissions (with special emphasis on admissions for heart failure, pulmonary oedema, and myocardial infarction), history of ischaemic heart disease (previous myocardial infarction, angina, or both), and history of hypertension and diabetes mellitus (insulin or non-insulin treated). Each questionnaire was evaluated immediately after completion and in case of any omissions or inconsistencies, the patients were contacted by telephone to ensure the highest possible quality of the data.
Measurements
Heart rate and blood pressure were measured and a 12 lead standard ECG was recorded and evaluated according to the Minnesota code.18 From predefined criteria (absence of arrhythmias, ST deviations, Q waves, hypertrophy, and abnormal LV axis), each ECG was classified as either normal or abnormal.
Furthermore, all participants underwent apical four and two chamber and apical long axis two dimensional echocardiography. LV systolic function was evaluated off-line in a blinded fashion by two experienced and independent observers by 16 segment wall motion score index. Normal investigations obtained a wall motion score of 2.0. By multiplying the wall motion score by 30, LV ejection fraction (LVEF) was calculated.19 Consequently LVEF ⩽ 55% reflects abnormal wall motion. LVEF was determined as the average value obtained by the two observers.
Finally, blood samples were collected for determination of biochemical markers. Plasma concentrations of NT-proBNP were measured by a novel, highly sensitive and specific immunoassay based on a sandwich format with unextracted EDTA plasma. The sensitivity of the assay was < 3.0 pmol/l and the intra-assay and inter-assay coefficients of variation were 1.3% and 4.8%, respectively.20
Mortality data were obtained from the Danish Central Personal Register, which registers all deaths in Denmark within two weeks. Data on admissions for heart failure and other major cardiovascular diseases were extracted from the National Danish Patient Register based on the discharge diagnosis classified according to the International classification of diseases, 10th revision (ICD-10).21 Heart failure admissions were defined as a primary discharge diagnosis of I50 and other major cardiovascular diseases were defined as I10–I15, I20–I25, I34–I36, I42.1–2,6–9, I46, and I49.9.
Statistical analysis
Normal data distribution was verified with histograms and normal plots. The plasma concentration of NT-proBNP had a log normal distribution and was consequently logarithmically (log10) transformed. Two-sample t tests between mean values of variables in groups with and without systolic heart failure were performed in case of normal distribution of data; alternatively Mann-Whitney tests were used. Dichotomous data were analysed by continuity adjusted χ2 tests. Plasma concentrations of NT-proBNP in different systolic heart failure groups were compared by analysis of variance. Potential markers for the diagnosis of systolic heart failure were analysed by multiple logistic regression analysis with backward elimination. Subsequently, receiver operating characteristic (ROC) curves for the ability of NT-proBNP to detect systolic dysfunction, as well as to diagnose systolic heart failure, were calculated. The optimum combination of sensitivity and specificity, defined as the point on the ROC curve closest to the upper left corner of the diagram, were derived together with the area under the curve (AUC). The ROC curve derived optimum NT-proBNP cut point concentration was subsequently used to calculate positive and negative predictive values. Potential independent prognostic markers for mortality, congestive heart failure admissions, and other cardiac admissions were evaluated by Cox proportional hazards analyses (time to first event). The study population was divided in two by the median NT-proBNP concentration. Kaplan-Meier curves for total mortality, congestive heart failure admissions, and other cardiac admissions were calculated in the two groups and differences between groups were assessed with the log rank test. All tests were two sided and a significance level of 5% was used. All tests were done with SAS (SAS Institute Inc, Cary, North Carolina, USA).
RESULTS
Demographic data
Table 1 gives demographic and clinical characteristics, as well as fundamental measurements.
Table 1.
Baseline characteristics and drug treatment
| Characteristics | |
| Demographic data | |
| Male/female | 290 (43%)/382 (57%) |
| Age (years) | |
| 50–59 | 174 (26%) |
| 60–69 | 204 (30%) |
| 70–79 | 174 (26%) |
| ⩾ 80 | 120 (18%) |
| Median (range) | 68.1 (51.2–91.3) |
| Current daily smoker | 237 (35%) |
| Clinical history | |
| Dyspnoea grade* | |
| 2 | 84 (13%) |
| 3 | 51 (8%) |
| 4 | 47 (7%) |
| 5 | 14 (2%) |
| 6 | 23 (3%) |
| Total | 219 (33%) |
| Ankle oedema | 155 (23%) |
| Ischaemic heart disease | 96 (14%) |
| Hypertension | 169 (25%) |
| Angina | 83 (12%) |
| Diabetes mellitus | |
| Insulin treated | 7 (1%) |
| Non-insulin treated | 36 (5%) |
| Measurements | |
| Atrial fibrillation | 24 (4%) |
| Heart rate (beats/min) | 75.4 (13.4) |
| Systolic blood pressure (mm Hg) | 144 (23.2) |
| Diastolic blood pressure (mm Hg) | 86.6 (12.2) |
| Ejection fraction | |
| ⩽ 50% | 77 (11%) |
| ⩽ 45% | 58 (9%) |
| ⩽ 40% | 38 (6%) |
| ⩽ 35% | 21 (3%) |
| Median (range) | 60 (10–60) |
| NT-proBNP (pmol/l)† | 35.7 (32.8 to 38.9) |
| Abnormal ECG‡ | 197 (30%) |
| Drug treatment | |
| Loop diuretics | 37 (6%) |
| Other diuretics | 82 (12%) |
| ACE inhibitor | 42 (6%) |
| Angiotensin II blocker | 12 (2%) |
| β Blocker | 30 (5%) |
| Calcium antagonist | 39 (6%) |
| Nitrates | 26 (4%) |
| Digitalis | 25 (4%) |
| Aspirin | 112 (17%) |
Data are numbers (%) or mean (SD).
ACE, angiotensin converting enzyme; NT-proBNP, N-terminal pro-brain natriuretic peptide.
*World Health Organization classification of dyspnoea (grade 2, dyspnoea when vacuum cleaning or climbing stairs to the second floor; grade 3, when walking on an even road; grade 4, at minimum exertion; grade 5, orthopnoea; grade 6, at rest).
†Geometric mean concentration and 95% confidence interval.
‡Normal ECG with absence of arrhythmias, ST deviations, Q waves, hypertrophy, and abnormal left ventricular axis according to the Minnesota code.
When comparing our study population with the background population of Copenhagen, there was no evidence of sex difference in sampling (Mantel-Haenszel male to female proportion (over the four ages groups) was 1.1 (NS)) and no evidence of heterogeneity over age groups (NS).
There was no difference in admission rates before sampling between the total invited sample and the background population (NS), nor was there any difference within the total invited sample between participants and non-participants (NS). During the year after sampling, the admission rate was significantly increased for the total invited sample (p = 0.03) with no significant difference between participants and non-participants (NS).
The participants had a one year mortality very close to that of the background population, whereas non-participants had a more than three times higher one year mortality than the participants (p < 0.001).
Detection of LV systolic dysfunction
To evaluate NT-proBNP as a marker for LV systolic dysfunction, ROC curves were calculated for the ability of NT-proBNP to detect LVEF ⩽ 50%, ⩽ 45%, ⩽ 40%, and ⩽ 35% (table 2).
Table 2.
Diagnostic value of NT-proBNP in detecting left ventricular systolic dysfunction
| LVEF cut off | Sensitivity/specificity | PPV/NPV | AUC | NT-proBNP (pmol/l)* | Diagnoses made per 100 screened | Diagnoses missed per 100 screened | False positives per 100 screened |
| 50% (n = 77) | 0.70/0.63 | 0.20/0.94 | 0.70 | 41.5 | 8.02 | 3.44 | 32.8 |
| 45% (n = 58) | 0.74/0.64 | 0.16/0.96 | 0.73 | 43.3 | 6.39 | 2.24 | 32.9 |
| 40% (n = 38) | 0.76/0.67 | 0.12/0.98 | 0.79 | 48.9 | 4.30 | 1.36 | 31.1 |
| 35% (n = 21) | 0.76/0.85 | 0.14/0.99 | 0.83 | 100.5 | 2.38 | 0.75 | 14.5 |
AUC, area under the curve; LVEF, left ventricular ejection fraction; NPV, negative predictive value; PPV, positive predictive value.
*NT-proBNP concentration corresponding to optimal combination of sensitivity and specificity.
Diagnosis of systolic heart failure
With the aim of evaluating the diagnostic potential of NT-proBNP in clinical heart failure, subjects were classified as having or not having systolic heart failure. Systolic heart failure was the diagnosis for patients with symptoms of heart failure in the form of dyspnoea or ankle oedema and with objective evidence of heart failure in the form of impaired systolic function by echocardiography (LVEF ⩽ 50%). These criteria are consistent with those set out by the European Society of Cardiology.9 LVEF ⩽ 50% was chosen as the main cut off for LV systolic dysfunction, as we aimed at assessing the diagnostic and prognostic utility of the marker in mild systolic heart failure. However, results with different cut offs (LVEF ⩽ 45%, ⩽ 40%, and ⩽ 35%) are also given. Stratified by these criteria, 623 patients were categorised as normal, whereas 49 (7% of the study population and 64% of patients with LVEF ⩽ 50%) met the criteria for systolic heart failure (table 3).
Table 3.
Baseline characteristics in normal participants and in patients with systolic heart failure
| Normal (n = 623) | Systolic heart failure* (n = 49) | p Value† | |
| Male sex | 42% | 55% | NS |
| Median (range) age (years) | 67.1 (51.2–91.3) | 76.3 (51.8–88.7) | <0.0001 |
| Mean (SD) heart rate(beats/min) | 75.2 (13.1) | 77.7 (16.3) | NS |
| Mean (SD) systolic blood pressure (mm Hg) | 143 (22.9) | 146 (27.0) | NS |
| Mean (SD) diastolic blood pressure (mm Hg) | 86.7 (12.0) | 84.7 (14.7) | NS |
| Median (range) LVEF (%) | 60 (15–60) | 40 (10–50) | <0.0001 |
| Mean (95% CI) NT-proBNP (pmol/l)‡ | 32.7 (30.1 to 35.5) | 112(73.1 to 170) | <0.0001 |
*Defined as symptoms of heart failure and LVEF of ⩽50%.
†p Value for differences between mean values in patients with and without systolic heart failure.
‡Geometric mean concentrations.
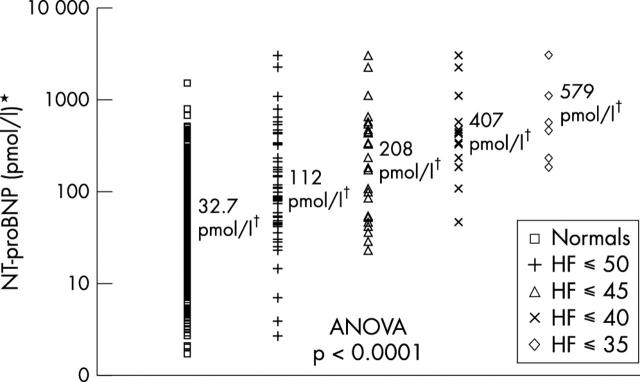
As fig 1 shows, the plasma concentration of NT-proBNP increased with worsening systolic heart failure (symptoms and LVEF ⩽ 50%, ⩽ 45%, ⩽ 40%, and ⩽ 35% (heart failure ⩽ 50/⩽ 45/⩽ 40/⩽ 35), respectively; p < 0.0001).
Figure 1.
Plasma concentrations of N-terminal pro-brain natriuretic peptide (NT-proBNP) in normal participants and in patients with symptoms of heart failure (HF) ⩽ 50/⩽ 45/⩽ 40/⩽ 35 and left ventricular ejection fraction (LVEF) ⩽ 50%, ⩽ 45%, ⩽ 40%, and ⩽ 35%, respectively. ANOVA, analysis of variance. *Log scale; †geometric mean concentrations.
Potential markers for heart failure ⩽ 50 were analysed in a multivariate model with history of hypertension, history of ischaemic heart disease, diabetes, age, sex, heart rate, systolic and diastolic blood pressures, abnormal ECG, and NT-proBNP. Log10 (NT-proBNP) (parameter estimate 1.43, odds ratio 4.17, p < 0.0001), abnormal ECG (parameter estimate 1.32, odds ratio 3.73, p = 0.0007), and history of ischaemic heart disease (parameter estimate 1.32, odds ratio 3.76, p = 0.0002) came out as independent markers.
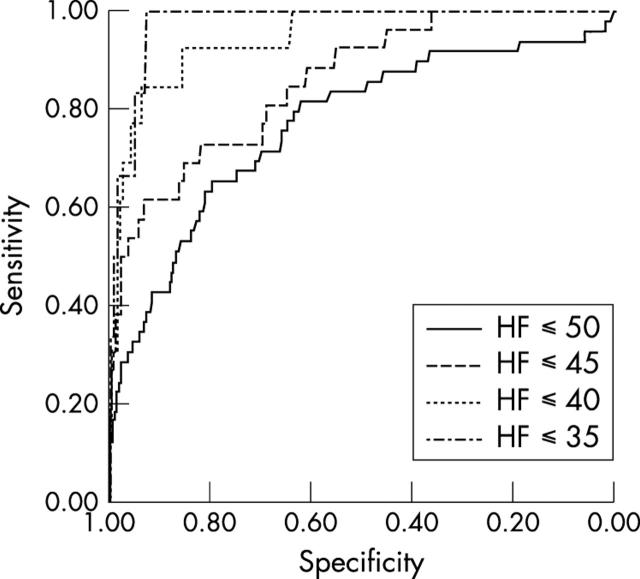
ROC curves were calculated for the ability of NT-proBNP to diagnose heart failure ⩽ 50, ⩽ 45, ⩽ 40, and ⩽ 35. For the diagnosis of heart failure ⩽ 40, the following results were obtained: sensitivity/specificity 0.92/0.86, positive predictive value /negative predictive value 0.11/1.00, AUC = 0.94, NT-proBNP = 106.7 pmol/l (fig 2, table 4).
Figure 2.
Receiver operating characteristic (ROC) curves for the ability of NT-proBNP to diagnose systolic HF ⩽ 50/⩽ 45/⩽ 40/⩽ 35, respectively, and LVEF ⩽ 50%/⩽ 45%/⩽ 40%/⩽ 35%, respectively.
Table 4.
Diagnostic value of NT-proBNP in detecting systolic heart failure
| Diagnosis* | SENS/SPEC | PPV/NPV | AUC | NT-proBNP (pmol/l)† | Diagnoses made per 100 screened | Diagnoses missed per 100 screened | False positives per 100 screened | |
| All (n = 672) | HF⩽50 (n = 49) | 0.65/0.80 | 0.20/0.97 | 0.77 | 72.8 | 4.74 | 2.55 | 18.5 |
| HF⩽40 (n = 13) | 0.92/0.86 | 0.11/1.00 | 0.94 | 106.7 | 1.78 | 0.15 | 13.7 | |
| Age > 70 years (n = 294) | HF⩽50 (n = 36) | 0.64/0.74 | 0.26/0.94 | 0.74 | 106.7 | 7.84 | 4.41 | 22.8 |
| HF⩽40 (n = 11) | 0.91/0.91 | 0.29/1.00 | 0.94 | 229.0 | 3.40 | 0.34 | 8.66 | |
| High risk medical history‡ (n = 246) | HF⩽50 (n = 34) | 0.68/0.72 | 0.28/0.93 | 0.73 | 72.8 | 9.40 | 4.42 | 24.1 |
| HF⩽40 (n = 9) | 0.89/0.80 | 0.14/0.99 | 0.90 | 106.7 | 3.26 | 0.40 | 19.3 |
*HF ⩽50/40 indicates patients with symptoms of HF and LVEF ⩽50/40%.
†NT-proBNP concentration corresponding to optimal combination of sensitivity and specificity.
‡Patients with a history of ischaemic heart disease (angina or previous myocardial infarction), history of hypertension, or diabetes mellitus.
High risk patients
A subgroup of patients at high risk of developing heart failure was defined as those with a history of ischaemic heart disease (angina, previous myocardial infarction, or both), history of hypertension, or diabetes mellitus (all self reported). One or more of these criteria were met by 246 patients (median (range) age 70.6 (51.2–90.8) years, geometric mean (95% confidence interval NT-proBNP 47.9 (41.4 to 55.4) pmol/l). Of these, 34 patients (14%) had diagnosed heart failure ⩽ 50.
A second high risk group of patients aged > 70 years (n = 294, median (range) age 78.5 (70.0–91.3) years, geometric mean (95% confidence interval) NT-proBNP 69.5 (61.9–78.0) pmol/l) was defined, in which 36 patients (12%) had diagnosed heart failure ⩽ 50.
The diagnostic power of NT-proBNP for systolic heart failure in these high risk groups was evaluated by ROC curve analyses and was, apart from significantly higher NT-proBNP cut off concentrations, only slightly inferior to its performance in the undivided population (table 4).
Prognosis
After the examination, patients were followed up for a median (range) of 805 (60–1171) days. Potential independent prognostic markers for total mortality, congestive heart failure admissions, and other cardiac admissions (history of hypertension, history of ischaemic heart disease, diabetes, age, sex, dyspnoea, ankle oedema, heart rate, systolic and diastolic blood pressures, abnormal ECG, LVEF, and NT-proBNP) were evaluated by Cox proportional hazards analyses.
Log10 (NT-proBNP) (hazard ratio (HR) 5.70, p < 0.0001) and male sex (HR 3.10, p = 0.004) were identified as independent markers for mortality. Log10 (NT-proBNP) (HR 13.83, p < 0.0001), dyspnoea (HR 1.45, p = 0.003), and male sex (HR 2.71, p = 0.03) came out as independent markers for congestive heart failure admissions. Lastly, log10 (NT-proBNP) (HR 3.69, p < 0.0001), abnormal ECG (HR 2.56, p = 0.003), and history of ischaemic heart disease (HR 1.90, p = 0.03) were identified as independent markers for other cardiac admissions, eliminating LVEF from all prognostic models.
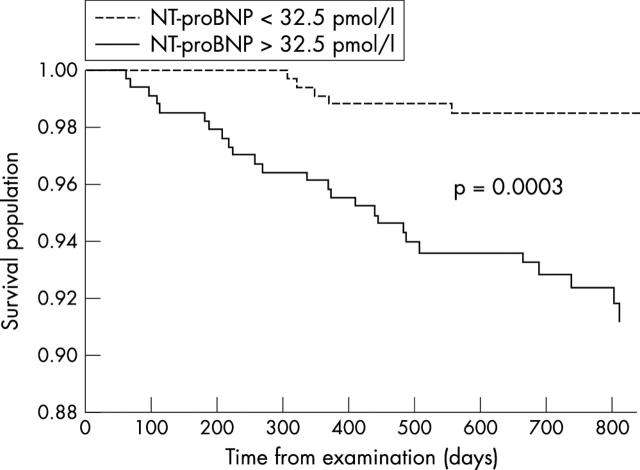
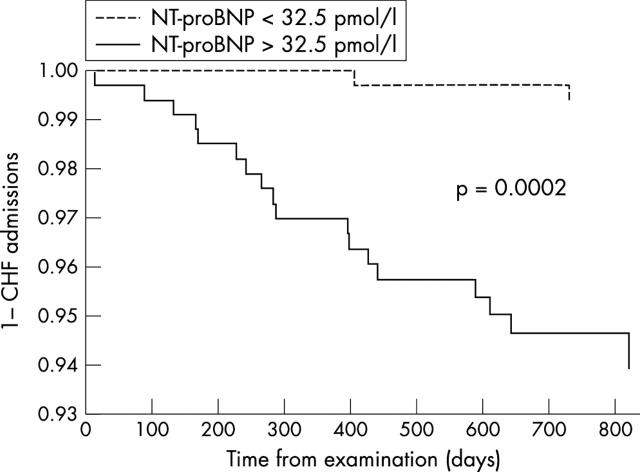
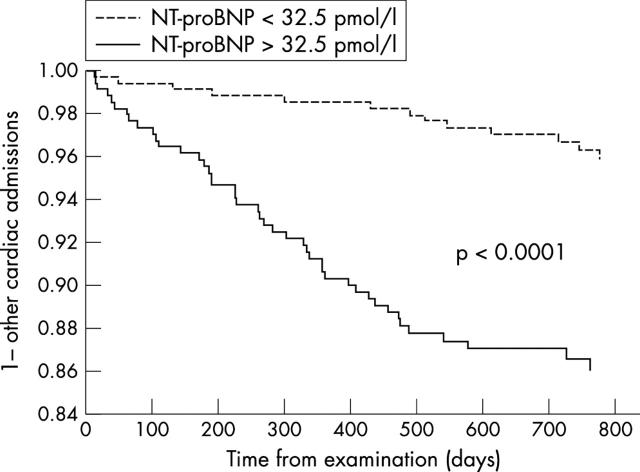
All-cause mortality (26 v 6, p = 0.0003) (fig 3), congestive heart failure admissions (18 v 2, p = 0.0002) (fig 4), and admissions for other cardiac causes (44 v 13, p = 0.0001) (fig 5) were significantly higher for patients with NT-proBNP > 32.5 pmol/l (median study concentration) than for patients with NT-proBNP < 32.5 pmol/l.
Figure 3.
Kaplan-Meier survival curves for patients with NT-proBNP plasma concentrations > 32.5 pmol/l and with NT-proBNP concentrations < 32.5 pmol/l (study median NT-proBNP concentration).
Figure 4.
Kaplan-Meier curves for the first admission to hospital for congestive heart failure (CHF) for patients with NT-proBNP plasma concentrations > 32.5 pmol/l and with NT-proBNP concentrations < 32.5 pmol/l (study median NT-proBNP concentration).
Figure 5.
Kaplan-Meier curves for the first admission to hospital for cardiac causes other than CHF for patients with NT-proBNP plasma concentrations > 32.5 pmol/l and with NT-proBNP concentrations < 32.5 pmol/l (study median NT-proBNP concentration).
DISCUSSION
This is the first population based study to confirm that the plasma concentration of NT-proBNP is raised in patients with LV systolic dysfunction and symptoms of heart failure and that NT-proBNP is a strong predictor of death and cardiac morbidity—superior to LVEF evaluated by high quality echocardiography. In addition, we have shown that in a random, age controlled sample of the general population, the plasma concentration of NT-proBNP can detect patients with systolic heart failure, consistent with the European Society of Cardiology criteria,9 with a high sensitivity and specificity.
Screening for initial stages of any disease, enabling earlier treatment and, theoretically, a greater reduction in morbidity and mortality, is a valuable medical tool. Screening for heart failure in the population seems to meet all of the well established criteria for a successful individual screening programme.22,23 The condition sought should be the precursor of an important health problem, which is certainly the case for heart failure, as it is both common1,3,4 and serious24 and it poses a significant economic burden to society.25 The natural history of the disease has to be well understood, which is also true for heart failure, as many studies have confirmed the eventual progression of myocardial damage of a certain extent to chronic heart failure, often through a phase of asymptomatic, potentially treatable LV dysfunction.26,27 The disease should be effectively treatable, which is the case for heart failure, as large scale trials have confirmed the effectiveness of angiotensin converting enzyme inhibitors,6 β blockers,7 and spironolactone8 in increasing the survival of patients with chronic heart failure. Lastly, and fundamental to any screening program, is the availability of a suitable diagnostic test.
Until now, systolic heart failure has been diagnosed by a combination of a clinical evaluation and some objective measure of LV systolic function. Clinical evaluation is unreliable and inherently incapable of detecting the asymptomatic precursor.28 Echocardiography, the predominant method of evaluating LV systolic function, is both observer dependent and somewhat imprecise, as the technique is most often based on assumptions of LV shape not fulfilled in most patients with LV damage. In addition, although population screening with echocardiography may provide a solution, it would be very labour intensive and probably not cost effective. Clearly, a cheap, simple, and precise biochemical screening option would be more attractive.
In our age controlled sample of the general population, NT-proBNP came out as a powerful diagnostic marker for systolic heart failure with a strength similar to that of other well established screening methods, such as prostate specific antigen for detection of prostate cancer (AUC = 0.94),29 and superior to mammography for breast cancer (AUC = 0.85)30 and Papanicolaou smears for cervical cancer (AUC = 0.70),31 suggesting that NT-proBNP is a valid and acceptable test for systolic heart failure in a population screening setting. In addition, NT-proBNP is very stable in plasma and in whole blood and can be stored at room temperature for several days without significant degradation. The test should therefore prove very simple to handle in practice.13
Until now, very limited population based data on the diagnostic performance of biochemical markers in heart failure have been published, making it difficult to compare our results with previous studies. However, in one population based study by McDonagh and colleagues,17 the diagnostic performance of BNP to detect LVEF ⩽ 30% was very similar to our results, yielding an AUC of 0.88 in the undivided general population. In a highly selected group of 106 patients with new symptoms suspected by a general practitioner to be due to heart failure, Cowie and colleagues32 found that BNP identified patients who were subsequently determined to have heart failure with an AUC of 0.96.
Restriction of our analysis to patients at high risk of heart failure, in which the prevalence of undetected systolic heart failure was 14%, compared with 7% in the whole study population, only slightly reduced the diagnostic potential of NT-proBNP, yielding a higher detection rate with little loss in sensitivity. Similar results were found by McDonagh and colleagues,17 supporting the idea that targeting of high risk patients may be a more efficient population screening approach. This is of course true, as the detection rate would go up; but, on the other hand, some, presumably the younger patients who are probably likely to benefit the most from relevant medication, would risk being missed in the screening. In our study, 13 of 49 (27%) patients with systolic heart failure were younger than 70 years.
Our finding that NT-proBNP was an excellent predictor of all cause mortality and cardiac morbidity is in line with previous results from Richards and colleagues,33 who, in patients with known ischaemic heart failure and in postmyocardial infarction patients,12 found the plasma concentration of NT-proBNP to be of prognostic significance. Apart from underscoring the pivotal role of the marker in heart failure, our prognostic findings suggest that initially missing the diagnosis of heart failure in patients with borderline NT-proBNP may not be crucial, as the prognosis of patients with NT-proBNP below the median is very good, even in the absence of relevant treatment. This view is further supported by the findings of Troughton and colleagues,34 who in patients with known heart failure showed that a reduction in the plasma concentration of NT-proBNP by standard heart failure medication was associated with an improved prognosis.
Study limitations
As shown in the results section, the non-responders were more ill than the participants. This tends to underestimate the true prevalence of heart failure, which means that some patients with severe heart failure have probably not been included in the study, especially among the elderly. This is likely to have given a less favourable result on the utility of the test. The number of events available for assessment of the prognostic utility of NT-proBNP was rather low. Even though the statistical analysis takes this into account, the prognostic data should be interpreted with caution.
Conclusions
Taken together, our results suggest that NT-proBNP may be useful as a screening tool for systolic heart failure in a general population screening setting.
Acknowledgments
The study was funded by F Hoffmann-La Roche Ltd, Basel, Switzerland; Merck Sharp & Dohme, Glostrup, Denmark; The Research Fund of the Copenhagen Hospital Corporation, Copenhagen, Denmark; Sophus Jacobsen and Wife Astrid Jacobsen’s Fund, Copenhagen, Denmark; Arvid Nilsson’s Fund, Copenhagen, Denmark; Leo Pharmaceutical’s Research Fund, Copenhagen, Denmark; Svend Hansen and Wife Ina Hansen’s Fund, Copenhagen, Denmark; Lykkefeldt’s Fund, Copenhagen, Denmark; Ove Villiam Buhl Olesen and Wife Edith Buhl’s Memorial Fund, Copenhagen, Denmark; Elisabeth M Schlinsog’s Fund, Copenhagen, Denmark.
Abbreviations
AUC, area under the curve
BNP, brain natriuretic peptide
HR, hazard ratio
LV, left ventricular
LVEF, left ventricular ejection fraction
NT-proBNP, N-terminal pro-brain natriuretic peptide
ROC, receiver operating characteristic
REFERENCES
- 1.Hedberg P, Lonnberg I, Jonason T, et al. Left ventricular systolic dysfunction in 75-year-old men and women. A population-based study. Eur Heart J 2001;22:676–83. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population: the Rotterdam Study. Eur Heart J 1999;20:447–55. [PubMed] [Google Scholar]
- 3.Cowie MR, Wood DA, Coats AJ, et al. Incidence and aetiology of heart failure: a population-based study. Eur Heart J 1999;20:421–8. [DOI] [PubMed] [Google Scholar]
- 4.McDonagh TA, Morrison CE, Lawrence A, et al. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet 1997;350:829–33. [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, MacIntyre K, Hole DJ, et al. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001;3:315–22. [DOI] [PubMed] [Google Scholar]
- 6.Anon. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD investigators. N Engl J Med 1991;325:293–302. [DOI] [PubMed] [Google Scholar]
- 7.Anon. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 1999;353:2001–7. [PubMed] [Google Scholar]
- 8.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med 1999;341:709–17. [DOI] [PubMed] [Google Scholar]
- 9.Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001;22:1527–60. [DOI] [PubMed] [Google Scholar]
- 10.Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997;96:509–16. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K, Burnett JCJ, Jougasaki M, et al. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension 1996;28:988–94. [DOI] [PubMed] [Google Scholar]
- 12.Richards AM, Nicholls MG, Yandle TG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation 1998;97:1921–9. [DOI] [PubMed] [Google Scholar]
- 13.Downie PF, Talwar S, Squire IB, et al. Assessment of the stability of N-terminal pro-brain natriuretic peptide in vitro: implications for assessment of left ventricular dysfunction. Clin Sci (Colch) 1999;97:255–8. [PubMed] [Google Scholar]
- 14.Richards AM, Crozier IG, Yandle TG, et al. Brain natriuretic factor: regional plasma concentrations and correlations with haemodynamic state in cardiac disease. Br Heart J 1993;69:414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magga J, Vuolteenaho O, Tokola H, et al. B-type natriuretic peptide: a myocyte-specific marker for characterizing load-induced alterations in cardiac gene expression. Ann Med 1998;30(suppl 1):39–45. [PubMed] [Google Scholar]
- 16.McDonagh TA, Cunningham AD, Morrison CE, et al. Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart 2001;86:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998;351:9–13. [DOI] [PubMed] [Google Scholar]
- 18.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ 1968;56:1–188. [PubMed] [Google Scholar]
- 19.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- 20.Karl J, Borgya A, Gallusser A, et al. Development of a novel, N-terminal-proBNP (NT-proBNP) assay with a low detection limit. Scand J Clin Lab Invest Suppl 1999;230:177–81. [PubMed] [Google Scholar]
- 21.Anon. International statistical classification of disease and health related problems, 10th rev. Geneva: World Health Organization, 1992.
- 22.Holland WW. Screening: reasons to be cautious. BMJ 1993;306:1222–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLean CD. Principles of cancer screening. Med Clin North Am 1996;80:1–14. [DOI] [PubMed] [Google Scholar]
- 24.Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–15. [DOI] [PubMed] [Google Scholar]
- 25.Stewart S, Jenkins A, Buchan S, et al. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail 2002;4:361–71. [DOI] [PubMed] [Google Scholar]
- 26.Anon Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med 1992;327:685–91. [DOI] [PubMed] [Google Scholar]
- 27.Vasan RS, Larson MG, Benjamin EJ, et al. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med 1997;336:1350–5. [DOI] [PubMed] [Google Scholar]
- 28.Remes J, Miettinen H, Reunanen A, et al. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 1991;12:315–21. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen SJ, Bergstralh EJ, Guess HA, et al. Predictive properties of serum-prostate-specific antigen testing in a community-based setting. Arch Intern Med 1996;156:2462–8. [PubMed] [Google Scholar]
- 30.Swets JA, Getty DJ, Pickett RM, et al. Enhancing and evaluating diagnostic accuracy. Med Decis Making 1991;11:9–18. [DOI] [PubMed] [Google Scholar]
- 31.Krane JF, Granter SR, Trask CE, et al. Papanicolaou smear sensitivity for the detection of adenocarcinoma of the cervix: a study of 49 cases. Cancer 2001;93:8–15. [PubMed] [Google Scholar]
- 32.Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet 1997;350:1349–53. [DOI] [PubMed] [Google Scholar]
- 33.Richards AM, Doughty R, Nicholls MG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. J Am Coll Cardiol 2001;37:1781–7. [DOI] [PubMed] [Google Scholar]
- 34.Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000;355:1126–30. [DOI] [PubMed] [Google Scholar]