Among patients undergoing coronary angiography because of angina typical enough to suggest coronary artery disease, 10–30% are found to have “normal” or “near normal” epicardial coronary arteries at angiography.
A group of these patients presents features of “cardiac syndrome X”, which is typically characterised by:
predominantly effort induced angina
ST segment depression suggestive of myocardial ischaemia during spontaneous or provoked angina
normal coronary arteries at angiography
absence of spontaneous or provoked epicardial coronary artery spasm
absence of cardiac (for example, hypertrophic or dilated cardiomyopathy) or systemic (for example, hypertension, diabetes) diseases potentially associated with microvascular dysfunction.
However, several groups of patients presenting with angina pectoris and normal coronary arteries do not fall into the strict definition of syndrome X, including those with predominant rest angina, those with hypertension or diabetes, or those with lack of ischaemic-like ECG changes during angina. It is still largely unknown whether the pathogenesis of angina in these various subsets of patients is different from that of angina in patients with typical syndrome X. Nevertheless, patients with typical and non-typical syndrome X have frequently been pooled together, thus making comparisons among different studies rather problematic. Therefore, a consensus document on the definition, classification, and management of patients with angina and normal coronary arteries would be very useful.
PATHOGENETIC MECHANISMS
Syndrome X is characterised by two major abnormalities, which may combine variously to determine the individual clinical picture: (1) coronary microvascular dysfunction; and (2) abnormal cardiac pain sensitivity.
Microvascular dysfunction
Since its first description it was suggested that, in syndrome X, angina is caused by myocardial ischaemia determined by a dysfunction of small resistance coronary artery vessels (< 500 μm) not visible at coronary angiography, a condition defined as “microvascular angina”.1
The occurrence of myocardial ischaemia in these patients is indicated by transient ST segment depression and reversible perfusion defects on nuclear imaging during anginal pain, either spontaneous or induced by exercise or pharmacological stress tests.2 Furthermore, metabolic evidence of stress induced myocardial ischaemia, including transmyocardial lactate production, coronary sinus oxygen desaturation, and pH reduction, as well as myocardial high energy phosphate depletion on nuclear magnetic resonance,3 have been documented in about 20% of patients with angina and normal coronary arteries.
Support for the notion that microvascular dysfunction is the cause of syndrome X derives from studies assessing coronary flow response to vasoactive stimuli. These studies have indeed shown an impairment of both endothelium dependent (for example, in response to pacing, acetylcholine) and endothelium independent (for example, in response to dipyridamole, papaverine) coronary vasodilation, using different techniques (for example, thermodilution, intracoronary Doppler recording, positron emission tomography, magnetic resonance, etc) for the measurement of coronary blood flow.4 Furthermore, coronary vasoconstrictor stimuli (for example, ergonovine, cold pressor test, hyperventilation, handgrip, and acetylcholine) have been found to induce symptoms and/or signs of myocardial ischaemia in a proportion of patients.
In contrast, other studies failed to find evidence of either microvascular dysfunction or metabolic abnormalities typical of myocardial ischaemia during stress tests.5 More importantly, echocardiographic studies have consistently failed to provide evidence of transient regional wall motion abnormalities during angina and transient ST segment depression, thus questioning the ischaemic, or even cardiac, origin of syndrome X.5,6
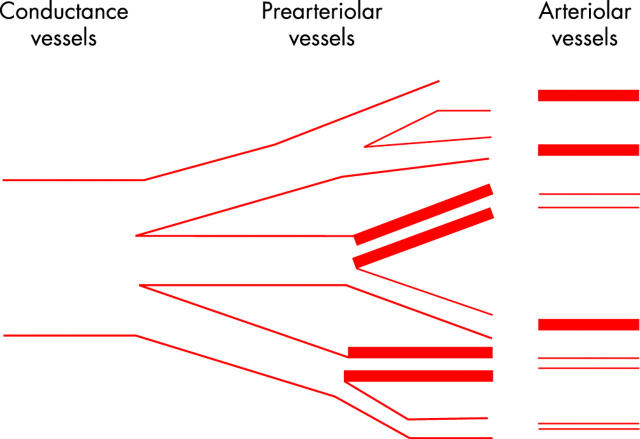
In order to reconcile these apparently contradictory findings, Maseri and colleagues2 proposed that coronary microvascular dysfunction might be limited to pre-arterioles, which are functionally defined as vessels of 100–500 μm in diameter, interposed between large epicardial arteries and arterioles. According to this hypothesis, the coronary pre-arteriolar dysfunction is scattered throughout the myocardium. Thus inappropriate constriction and/or impaired pre-arteriolar dilation in response to metabolic or pharmacological arteriolar dilatation would cause ischaemia in small myocardial regions surrounded by regions with a normal microvascular function (fig 1). The latter, however, exhibit normal, or even compensatory increased myocardial contractility, thus making it difficult to detect the regional (and global) contractile abnormalities. Furthermore, in this setting, metabolic markers of ischaemia released from small myocardial regions supplied by dysfunctioning pre-arterioles would also be diluted by blood draining normal myocardial regions, thus making their detection difficult using traditional approaches.
Figure 1.
Proposed pathophysiological mechanism of syndrome X. Coronary microvascular dysfunction might be limited to pre-arterioles, functionally defined as 100–500 μm vessels interposed between large epicardial arteries and arterioles (responsible for metabolic regulation of coronary blood flow), supplying small myocardial regions scattered throughout the myocardium. An inappropriate constriction and/or impaired pre-arteriolar dilation, in response to metabolic or pharmacological arteriolar dilatation, would cause ischaemia in small myocardial regions surrounded by areas with a normal microvascular function, exhibiting normal, or even compensatory increased myocardial contractility, which would limit regional (and global) contractile abnormalities. In this setting, metabolic markers of ischaemia released from small myocardial areas would be diluted in the coronary blood circulation, thus making their detection difficult using traditional approaches. Of note, a sustained compensatory release of adenosine from myocardial ischaemic regions might contribute to limit the degree of ischaemia. Yet adenosine might by itself induce chest pain (because of its algogenic effect mediated by A1 receptor stimulation on cardiac pain receptors), ischaemia-like ST changes (because of its electrophysiological effect mediated by A1 receptor stimulation on myocardial fibres), and even exacerbate subendocardial ischaemia through a steal mechanism. Modified from Maseri et al.2
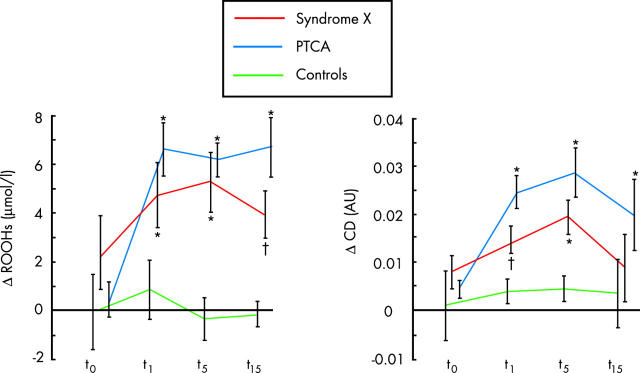
A sustained compensatory release of adenosine from myocardial ischaemic regions might contribute to limit the degree of ischaemia. Yet adenosine might by itself induce chest pain (because of its algogenic effect mediated by A1 receptor stimulation on cardiac pain receptors), ischaemia-like ST segment changes (because of its electrophysiological effect mediated by A1 receptor stimulation on myocardial fibres), and even exacerbate subendocardial ischaemia through a steal mechanism. This pathophysiological hypothesis is supported by studies with positron emission tomography, which have shown increased heterogeneity of coronary blood flow among small myocardial regions, both at rest and in response to dipyridamole, in syndrome X patients, as compared to healthy controls.7 Furthermore, convincing evidence of myocardial ischaemia in syndrome X has recently been given by Buffon and colleagues,8 who have shown a consistently sustained increase of lipid hydroperoxides and conjugated dienes—two markers of ischaemia–reperfusion oxidative stress—in the great cardiac vein blood after atrial pacing induced ST segment depression and angina. Indeed, oxidative stress products are much more sensitive than conventional metabolic markers in revealing ischaemia, as shown in clinical studies in which ischaemia is caused by balloon occlusion in the setting of coronary angioplasty (fig 2).
Figure 2.
Cardiac production of oxidative hydroperoxides (ROOHs) (left panel) and conjugated dienes (CD) (right panel) after atrial pacing in patients with syndrome X. The production of these oxidative lipoperoxide products during pacing, in the presence of ST segment depression and angina, is indicative of an ischaemia–reperfusion damage similar to that observed during balloon vessel occlusion in patients with obstructive coronary stenosis undergoing coronary angioplasty. There is no evidence of oxidative product release during atrial pacing in subjects without any history of angina pectoris (controls). Measures are obtained in blood samples from coronary sinus. t0 = base; t1, t5, t15 = 1, 5, and 15 minutes after atrial pacing or coronary angioplasty. *p < 0.01 and †p < 0.05 v baseline. Modified from Buffon et al.8
The causes of microvascular dysfunction in syndrome X have not yet been fully elucidated and are likely to be multiple. Structural abnormalities, mainly consisting of medial hypertrophy and/or fibrosis of arteriolar vessels, frequently associated with systemic hypertension, have been described in small series of patients.
A frequently reported abnormality is endothelial dysfunction, suggested by a decreased coronary flow response to acetylcholine, atrial pacing, and other endothelium mediated vasodilator stimuli, and believed to be caused by impaired nitric oxide (NO) release and/or activity. Decreased NO generation has been suggested by lower nitrate/nitrite systemic concentrations,9 although cGMP (the intracellular messenger for NO) release in the coronary circulation was not found to be decreased after atrial pacing in a group of syndrome X patients.10 Of note, an increased synthesis of asymmetric dimethylarginine, which is known to reduce the bioavailability of l-arginine for NO synthesis in endothelial cells, has recently been suggested to contribute to impaired NO activity in syndrome X patients.9 Several findings, however, suggest that endothelial dysfunction might cause not only impaired vasodilation, but also inappropriate vasoconstriction. Indeed, increased plasma concentrations of the powerful vasoconstrictor endothelin-1 (ET-1) have been reported in peripheral blood of syndrome X patients and have been shown to correlate with coronary microvascular dysfunction. Moreover, ET-1 has been found to increase in the coronary circulation of syndrome X patients in response to atrial pacing.10
Other studies, however, have also shown impairment of coronary microvascular dilation in response to adenosine, dipyridamole, and papaverine—that is, to endothelium independent stimuli—suggesting a possible primary smooth muscle cell abnormality.4
Enhanced sodium–hydrogen (Na+–H+) exchanger activity in cell membranes,11 consistently observed in syndrome X, represents a potential cause of microvascular dysfunction as it may induce cellular alkalinisation, thus resulting in increased susceptibility of smooth muscle cells to constrictor stimuli. Furthermore, enhanced Na+–H+ exchanger activity might be involved in several other typical features of syndrome X, including increased insulin resistance (associated with endothelial dysfunction), altered adrenergic activity, which may further favour microvascular dysfunction, and also enhanced pain perception.
More recently, a role for intracellular rho-kinase, which may enhance vasoconstriction in vascular smooth muscle cells by facilitating calcium overload, has also been suggested in patients with angina and normal coronary arteries.12
Finally, recent data suggest that low grade inflammation might also play a pathogenetic role in the microvascular dysfunction of patients with cardiac syndrome X. Indeed, recent studies have shown increased markers of inflammation in syndrome X patients, as compared to matched healthy controls, and Cosin-Sales and colleagues have also found a correlation between C reactive protein values and clinical and electrocardiographic indices of disease activity in patients with angina and normal coronary arteries.13
Abnormal cardiac pain perception
Several studies have consistently shown that patients with angina and normal coronary arteries exhibit enhanced pain perception. It is not definitely established, however, whether this feature is confined to stimuli originating from the heart or is rather generalised. The first hypothesis is supported by the results of a prospective, randomised, “sham” controlled study, in which we have shown that pain is elicited by low rate heart stimulation, in the absence of ST segment depression, during ventricular pacing, but not during atrial pacing.14
Another area of uncertainty is the site of the “neural” abnormality responsible for enhanced pain perception. Rosen and colleagues6 recently found activation of the right anterior insula cortex in syndrome X patients, but not in controls, during angina and ST changes induced by echocardiographic dobutamine stress test, in the absence of left ventricular wall motion abnormalities. These findings led the authors to suggest that syndrome X might be a “cortical pain syndrome”, resulting in a “top down” process which facilitates the transmission to the pain area of the cortex of stimuli which are usually blocked at subcortical level. However, hyperreactivity of cardiac pain receptors, abnormal transmission and/or modulation of pain signal at subcortical level(s), or a variable combination of all these abnormalities, might equally account for the abnormal activation of specific cerebral areas.
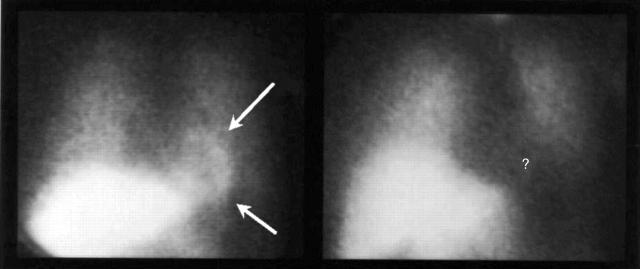
Regardless of its location, the cause of the neural abnormality is still poorly understood. We recently proposed a possible link between microvascular dysfunction and enhanced painful perception of cardiac stimuli. Based on the observation that most syndrome X patients exhibit pronounced functional abnormalities of cardiac adrenergic nerve fibres (fig 3),15 we hypothesised that repeated episodes of myocardial ischaemia might induce functional alterations in cardiac afferent nerve endings, resulting in increased reactivity to usually innocuous stimuli. Inflammatory or metabolic abnormalities might also adversely affect cardiac afferent nerve fibres. Notably, abnormal function of efferent cardiac adrenergic fibres might adversely influence microvascular function and coronary blood flow. It might be possible, however, that in some cases coronary microvascular dysfunction and abnormal pain perception represent independent abnormalities, and syndrome X becomes manifest because coronary microvascular abnormality happens to occur in patients with enhanced pain sensitivity. Regardless of its causes and mechanisms, enhanced cardiac pain perception is likely to play a key role in determining the frequent presence of invalidating symptoms in these patients.
Figure 3.
Radionuclide images obtained three hours after the injection of 123I-metaiodobenzylguanidine (MIBG) from a healthy subject (left panel) and from a patient with syndrome X (right panel). Cardiac MIBG uptake was normal (arrows) in the control subject, but it was totally absent (question mark) in the syndrome X patient, despite normal lung and liver MIBG uptake, consistent with a severe impairment of cardiac sympathetic function. Modified from Lanza et al.15
DIAGNOSIS
Among patients who present angina in the absence of transient ischaemic ECG changes and normal coronary arteries, non-cardiac causes of symptoms, such as musculoskeletal, psychiatric, and, particularly, gastrointestinal disorders must be excluded by appropriate diagnostic investigations. Furthermore, in patients with angina and normal coronary arteries, an intracoronary (or intravenous) ergonovine test should be performed, in particular when angina occurs predominantly at rest, in order to exclude occlusive coronary artery spasm.
Among patients fulfilling the strict definition of syndrome X, clinical features do not allow differentiation between those who will be found to have normal coronary arteries from those who will be found to have obstructive stenoses at coronary angiography. Angina may present, however, some clinical features which may suggest the presence of normal coronary arteries, including a prolonged (> 10 minutes) duration or a dull persistence after effort interruption, and an inconstant or slow response to sublingual nitrates. Furthermore, compared to patients with obstructive coronary artery disease, those with angina and normal coronary arteries are more frequently women often in a post-menopausal state.
Exercise test results do not help identify patients with syndrome X; indeed ST segment changes during exercise stress test are similar in patients with syndrome X and in those with angina and critical epicardial coronary stenoses. Furthermore, myocardial scintigraphy shows typical reversible myocardial perfusion defects in about a half of patients with syndrome X. Yet the induction of severe angina and ST segment changes during stress testing (dipyridamole, dobutamine, exercise), in the absence of regional and global left ventricular contractile abnormalities at echocardiography, strongly suggests a microvascular origin of symptoms.5,6 Repeat exercise testing after sublingual nitrates also may help to identify patients with microvascular dysfunction. Indeed, whereas short acting nitrate administration usually improves exercise test results in patients with epicardial coronary stenoses, it may not affect or may even worsen the results of exercise testing in syndrome X patients.16 These findings are in keeping with the variable response of chest pain to sublingual nitrates and with the evidence that short acting nitrates may paradoxically decrease coronary flow and ischaemic threshold during atrial pacing in these patients.
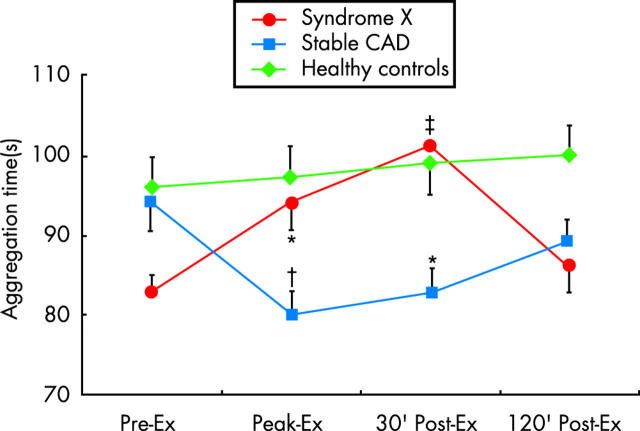
Finally, we have recently shown that the assessment of platelet aggregation before and after exercise testing may also help to identify syndrome X patients (fig 4).17 Indeed, in contrast to patients with obstructive coronary stenoses, who present increased platelet aggregation in response to exercise, syndrome X patients exhibit a reduction of platelet aggregation after exercise, possibly representing a protective effect against stress induced vascular events. Notably, a lengthening of the aggregation time ⩾ 10 seconds was detectable in patients with syndrome X only. These findings are probably explained, at least in part, by sustained compensatory release of adenosine (known to inhibit platelet aggregation).
Figure 4.
Changes in time to platelet aggregation induced by exercise in patients with obstructive coronary artery disease (CAD), in patients with syndrome X, and in matched healthy controls. Platelet aggregation, measured using the PFA-100 method, with collagen and adenosine diphosphate as activating stimuli, does not change in controls, but it increases in CAD patients. In contrast, platelet aggregation decreases in syndrome X patients. Ex, exercise. *p < 0.01, †p < 0.001, and ‡p < 0.0001 versus pre-exercise. Modified from Lanza et al.17
PROGNOSIS
All studies have consistently shown that among patients with angina and normal coronary arteries the occurrence of major cardiac events (death, myocardial infarction, cardiac heart failure) is similar to that observed in age and sex matched healthy controls.18 A progressive impairment of left ventricular function has been reported in a single study in a small number of patients presenting with angina and left bundle branch block, but these patients were probably affected by an initial form of dilated cardiomyopathy.
In spite of this excellent prognosis, several patients with angina and normal coronary arteries show persistence and even worsening of anginal symptoms over time. Anginal episodes may become more frequent, prolonged, poorly responsive to medical management, and may considerably restrict daily activities. Furthermore the severity of angina may lead to frequent non-invasive testing, to frequent hospital admissions for repeat coronary angiography, and also to frequent absence or even retirement from work. Thus, in these patients quality of life is often severely impaired. These features make syndrome X a socially and economically relevant cardiac disease.
TREATMENT
As the prognosis is excellent, the primary end point in the treatment of patients with angina and normal coronary arteries is symptom control. Accordingly, interventions found to have beneficial effects on symptoms should be preferred to treatment tested only on non-clinical end points, including ischaemic ECG changes or coronary flow response to vasoactive stimuli.
Interventions proposed in these patients are directed at either improving microvascular function or decreasing chest pain perception, in an attempt to counteract the accepted pathophysiological mechanisms of the syndrome. Unfortunately, most treatments have been evaluated in small numbers of patients and/or in uncontrolled trials, thus making it difficult to establish their real degree of effectiveness.
Traditional anti-ischaemic drugs remain the first line approach to these patients. Yet they frequently fail to achieve a satisfactory suppression of symptoms, thus stimulating the search for alternative therapeutic options.
Anti-ischaemic drugs
β Blockers
Some studies have reported that β blockers improve symptoms, exercise test results, and ST segment depression on Holter monitoring in patients with typical syndrome X. In a small randomised, controlled study we found that atenolol, but not amlodipine or isosorbide-5-mononitrate, achieved a significant, although mild, reduction in anginal symptoms. In general, β blocking agents should constitute the first line drug treatment in these patients, particularly when there is evidence of increased adrenergic tone (for example, high heart rate or decreased heart rate variability during 24 hour Holter monitoring, or rapid increase of heart rate and/or blood pressure during exercise).
Nitrates
Sublingual nitrates may be used to treat chest pain attacks in syndrome X patients. However, in about 50% of cases their effectiveness is limited. Long acting nitrates have only been assessed in our small trial, showing, on average, no significant effect on symptoms. As noted above, sublingual nitrates may facilitate ST segment changes induced by exercise in syndrome X patients, but this is not associated with any significant increased risk and, therefore, there is no contraindication to the use of sublingual nitrates to alleviate chest pain in these patients.
Calcium antagonists
In a controlled study, verapamil was shown to improve exercise test results and symptoms, but patients were selected according to a previous favourable symptomatic response to calcium antagonists. Diltiazem, but not propranolol, was found to improve exercise test results in one study, but it failed to improve coronary flow in another study. Among dihydropyridine drugs, nifedipine was found to improve coronary blood flow and exercise test results, but its administration was associated with worsening of symptoms in some patients. Favourable effects have been reported with nisoldipine, whereas, in the above mentioned study, we failed to observe an average beneficial effect of amlodipine on anginal symptoms.
Additional forms of treatment
Xanthine derivatives
Favourable effects on symptoms and exercise test results have been reported both with theophylline and bamiphylline, although the latter seems to be better tolerated. The beneficial effects of both compounds are probably caused by adenosine receptor blockade, which may result in both an anti-algogenic effect (due to the direct involvement of adenosine in cardiac pain generation) and in an anti-ischaemic effect (owing to a more favourable redistribution of coronary blood flow).
Chronic visceral pain inhibitors
Imipramine inhibits pain transmission from visceral tissues and was found to reduce anginal episodes in patients with angina and normal coronary arteries in two studies. However, quality of life was not significantly improved in another study, because of unpleasant side effects.
Oestrogens
As noted above, epidemiological studies show that syndrome X patients are often post-menopausal women, thus suggesting that oestrogen deficiency may have a pathogenic role. Notably, oestrogens cause endothelial mediated coronary vasodilation. In a randomised, placebo controlled trial, 17β oestradiol was found to determine a mild to moderate reduction of anginal episodes in post-menopausal women, although it failed to improve exercise test results.
α Antagonist drugs
These drugs might act by decreasing α mediated vasoconstriction; however, studies with prazosine and doxazosine (peripheral action) or clonidine (central action) did not achieve consistent clinical effects.
ACE inhibitors
Angiotensin converting enzyme (ACE) inhibitors have been proposed in the assumption that the renin–angiotensin system might play a role in causing microvascular dysfunction. Although there are limited data on these drugs, favourable effects have been reported on exercise test results, and on symptoms in small controlled trials. Accordingly, in a recent study in patients with angina and normal coronary arteries, enalapril has been found to have favourable effects on NO availability.9
Statins
Very recent data have shown that use of statins may improve angina symptoms and exercise tolerance in syndrome X patients, likely because of improvement of endothelial vasodilator function mediated by reduction of oxidative stress.19
Fatty acid oxidation partial inhibitors
Myocardial cells utilise both fatty acids and glucose as fuel, but glucose oxidation is more efficient. By switching cell metabolism toward glucose oxidation, these drugs should allow more work for the same amount of oxygen and delay the appearance of ischaemic suffering during stress conditions. Trimetazidine, a fatty acid oxidation partial inhibitor, was found to have beneficial effects on exercise test results in a small group of patients with syndrome X, but no effects were observed on symptoms and on exercise test results in another study. Thus the clinical utility of this drug is at least controversial.
ET-1, Na+–H+ exchanger, and rho-kinase inhibitors
The demonstration that ET-1 and Na+–H+ exchanger may be involved in the pathogenesis of syndrome X suggests that their specific antagonists (for example, bosentan and cariporide, respectively) are worth trying in randomised controlled trials. Similarly, the recent observation that the rho-kinase inhibitor fasudil may prevent vasoconstriction induced by acetylcholine in patients with microvascular angina suggests a possible use in these patients.
Angina pectoris and normal coronary arteries: key points.
- Typical cardiac syndrome X is characterised by:
- predominantly effort induced angina
- ST segment depression during spontaneous or provoked angina
- normal coronary arteries at angiography
- absence of epicardial coronary artery spasm
- absence of cardiac or systemic diseases potentially associated with microvascular dysfunction
Syndrome X results from two major pathogenetic components, which combine variously to determine the individual clinical pattern: coronary microvascular dysfunction and increased pain perception of cardiac stimuli
- In patients with angina pectoris, the diagnosis of syndrome X is suggested by:
- very variable duration of angina episodes
- inconstant or partial response to sublingual nitrates
- ST segment depression and angina in the absence of left ventricular wall motion abnormalities during pharmacological stress test
- earlier appearance of ST segment depression or angina on repeat exercise testing after sublingual nitrate administration
- decrease of ex-vivo platelet aggregation following exercise testing
Patients can be reassured about clinical outcome, as the prognosis of syndrome X is excellent as far as survival and occurrence of major cardiac events are concerned. Quality of life, however, may be severely affected by recurrent angina, thus making syndrome X a socially and economically relevant cardiac disease
Treatment of syndrome X should initially be based on β blockers. Yet anti-ischaemic drugs are frequently ineffective on symptom control. The addition of xanthines, ACE inhibitors, oestrogens, and imipramine may be helpful. Spinal cord stimulation can be considered in patients with angina refractory to multiple drug treatment
Electrical neuromodulation
Spinal cord stimulation modulates pain transmission from the heart to the brain and might improve myocardial ischaemia through inhibition of sympathetic efferent nerves. Spinal cord stimulation has been shown to improve exercise test results in one study in patients with typical syndrome X, and transcutaneous electrical nerve stimulation has been reported to improve coronary flow in another study. Furthermore, in a recent study we found that spinal cord stimulation was associated with a notable improvement of symptoms and quality of life in about 50% of a small group of patients with syndrome X refractory to other forms of treatment, in the absence of major complications. Thus, this approach should be considered in patients with angina refractory to multi-drug treatment.20
Stepwise approach
In patients with angina and normal coronary arteries, the response to treatment is rather unpredictable. Treatment, therefore, is necessarily empiric and requires an optimal interaction between caring physician and patient in an attempt to achieve optimal symptom control.
We usually use a β blocker as first line treatment and then we add a non-dihydropiridine (or even a dihydropiridine) calcium antagonist and/or a long acting nitrate, as a second line of treatment. When conventional anti-ischaemic treatment is not satisfactory, we usually add bamiphylline (600 mg twice a day). An ACE inhibitor, oestrogens (in post-menopausal women) and, according to recent data, a statin can be tried at any time. Occasionally, α blockers may also be tried, while a trial with imipramine (25 mg/day) should be reserved for the most symptomatic patients, in particular if signs of depression are apparent. In patients who remain severely symptomatic on multiple drug treatment we tend now to resort to spinal cord stimulation, which, in our early experience, results in a persistent symptomatic improvement in a sizeable proportion of patients with refractory angina.
Supplementary Material
REFERENCES
- 1.Cannon RO, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol 1988;61:1338–43. ▸ This study summarises the earlier evidence of coronary microvascular dysfunction in patients with angina and normal coronary arteries. [DOI] [PubMed] [Google Scholar]
- 2.Maseri A, Crea F, Kaski JC, et al. Mechanisms of angina pectoris in syndrome X. J Am Coll Cardiol 1991;17:499–506. ▸ This article reviews the evidence in favour and against an ischaemic origin of syndrome X. The authors then propose a novel pathogenetic hypothesis, which explains why transient myocardial ischaemia may occur in syndrome X in the absence of regional wall motion abnormalities and inconsistent detection of metabolic markers of ischaemia. [DOI] [PubMed] [Google Scholar]
- 3.Buchthal SD, den Hollander JA, Merz CNB, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 2000;342:829–35. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan A, Mullins PA, Taylor M, et al. Both endothelium-dependent and endothelium-independent function is impaired in patients with angina pectoris and normal coronary angiograms. Eur Heart J 1997;18:60–8. [DOI] [PubMed] [Google Scholar]
- 5.Cannon RO, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome X. Circulation 1992;85:883–92. ▸ This study reviews literature data which challenge the notion that myocardial ischaemia is actually responsible for chest pain and ST segment changes in syndrome X patients. [DOI] [PubMed] [Google Scholar]
- 6.Rosen SD, Paulesu E, Wise RJS, et al. Central neural contribution to the perception of chest pain in cardiac syndrome X. Heart 2002;87:513–9. ▸ This study shows that during dobutamine echocardiographic stress testing, angina and ST segment depression occurring in the absence of regional wall motion abnormalities are associated with activation of specific cortical regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galassi AR, Crea F, Araujo LI, et al. Comparison of myocardial blood flow in syndrome X and one-vessel coronary artery disease. Am J Cardiol 1993;72:134–42. ▸ A positron emission tomography study showing heterogeneous myocardial blood flow at rest and in response to dipyridamole in syndrome X, supporting the pathogenetic hypothesis proposed in reference 2. [DOI] [PubMed] [Google Scholar]
- 8.Buffon A, Rigattieri S, Santini SA, et al. Myocardial ischemia-reperfusion damage after pacing-induced tachycardia in patients with cardiac syndrome X. Am J Physiol Heart Circ Physiol 2000;279:H2627–33. ▸ A study which gives consistent evidence of stress induced myocardial ischaemia in patients with cardiac syndrome X, measuring stress induced oxidative stress productions, which are highly sensitive for ischaemia–reperfusion damage, in the coronary circulation. [DOI] [PubMed] [Google Scholar]
- 9.Chen JW, Hsu NW, Wu TC, et al. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol 2002;90:974–82. [DOI] [PubMed] [Google Scholar]
- 10.Lanza GA, Lüscher TF, Pasceri V, et al. Effects of atrial pacing on arterial and coronary sinus endothelin-1 levels in syndrome X. Am J Cardiol 1999;84:1187–91. [DOI] [PubMed] [Google Scholar]
- 11.Gaspardone A, Ferri C, Crea F, et al. Enhanced activity of sodium-lithium countertransport in patients with cardiac syndrome X: a potential link between cardiac and metabolic syndrome X. J Am Coll Cardiol 1998;32:2031–4. ▸ A study which shows an increase of sodium–hydrogen exchanger activity in syndrome X patients, potentially able to influence several features of syndrome X, including microvascular dysfunction, abnormal pain perception, and increased insulin resistance. [DOI] [PubMed] [Google Scholar]
- 12.Mohri M, Shimokawa H, Hirakawa Y, et al. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol 2003;41:15–9. [DOI] [PubMed] [Google Scholar]
- 13.Cosin-Sales J, Pizzi C, Brown S, et al. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. J Am Coll Cardiol 2003;41:1468–74. [DOI] [PubMed] [Google Scholar]
- 14.Pasceri V, Lanza GA, Buffon A, et al. Role of abnormal pain sensitivity and behavioral factors in determining chest pain in syndrome X. J Am Coll Cardiol 1998;31:62–6. [DOI] [PubMed] [Google Scholar]
- 15.Lanza GA, Giordano AG, Pristipino C, et al. Abnormal cardiac adrenergic nerve function in patients with syndrome X detected by [123I]metaiodobenzylguanidine myocardial scintigraphy. Circulation 1997;96:821–6. ▸ This study provides direct evidence of impaired cardiac sympathetic innervation in patients with syndrome X. [DOI] [PubMed] [Google Scholar]
- 16.Lanza GA, Manzoli A, Bia E, et al. Acute effects of nitrates on exercise testing in patients with syndrome X. Clinical and pathophysiological implications. Circulation 1994;90:2695–700. [DOI] [PubMed] [Google Scholar]
- 17.Lanza GA, Sestito A, Iacovella S, et al. Relation between platelet response to exercise and coronary angiographic findings in patients with effort angina. Circulation 2003;107:1378–82. ▸ A paper showing that, in contrast to patients with obstructive coronary artery stenoses, those with cardiac syndrome X present reduced platelet activation during stress induced ST segment depression and angina. [DOI] [PubMed] [Google Scholar]
- 18.Kaski JC, Rosano GMC, Collins P, et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol 1995;25:807–14. ▸ A prospective study confirming the excellent long term prognosis of syndrome X, but also the frequent relevant impairment of the quality of life. [DOI] [PubMed] [Google Scholar]
- 19.Pizzi C, Manfrini O, Fontana F, et al. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac syndrome X. Role of superoxide dismutase activity. Circulation 2004;109:53–8. [DOI] [PubMed] [Google Scholar]
- 20.Lanza GA, Sestito A, Sandric S, et al. Spinal cord stimulation in patients with refractory anginal pain and normal coronary arteries. Ital Heart J 2001;2:25–30. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.