Aortic intramural haematoma (IMH), a variant form of classic aortic dissection, has been accepted as an increasingly recognised and potentially fatal entity of acute aortic syndrome (AAS).1 In classic aortic dissection, flow communication occurs through a demonstrable primary intimal tear and blood flow propagation creates a so called “double channel aorta” with a true and false lumen. In IMH, it is believed that haemorrhage occurs within the aortic wall in the absence of initial intimal disruption. Thus, conventional aortography, which is useful for detection of intimal flap or double channel aorta in classic aortic dissection, failed to identify this disease entity and antemortem diagnosis of IMH was difficult.2 With recent advances and successful clinical introduction of various non-invasive imaging modalities for aortic pathology, such as contrast enhanced x ray computed tomography (CT), magnetic resonance imaging (MRI), and transoesophageal echocardiography (TOE), the clinical significance of IMH can be truly estimated. In this presentation, I would like to focus on the characteristic imaging features of IMH by these modalities and difficult scenarios for diagnosis.
DIAGNOSTIC CRITERIA
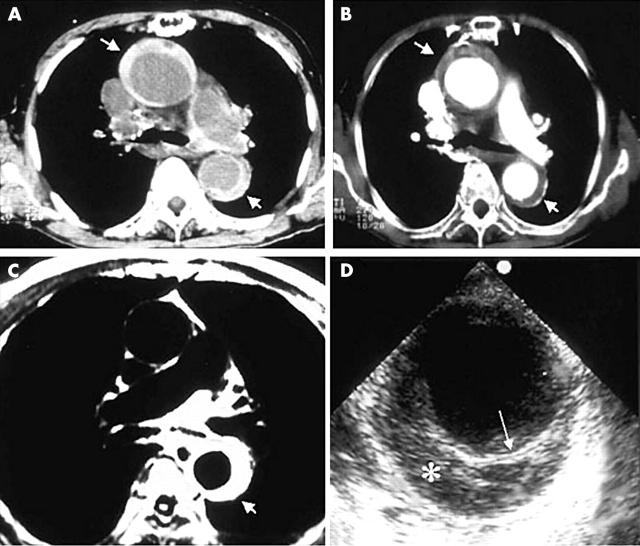
Non-invasive radiological techniques, CT and MRI, were first used for diagnosis of IMH—“aortic dissection without intimal rupture”.3 In CT, demonstration of continuous, usually crescentic, high attenuation areas along the aortic wall without intimal flap is characteristic before contrast injection (fig 1A), which fail to be enhanced after injection of contrast medium (fig 1B). Crescentic aortic wall thickening without intimal flap or tear is also very easily detected by MRI; the signal intensity of the thickened aortic wall might be variable, dependent on the amount of methaemoglobin formation within the haematoma (fig 1C). TOE is also useful to demonstrate circumferential or crescentic aortic wall thickening without intimal tear4; displacement of intimal calcification caused by accumulation of blood within the aortic media can be demonstrated, which is useful for differential diagnosis (fig 1D). Initial investigators used aortic wall thickness > 7 mm as a diagnostic criteria of IMH.4 According to our own data, the mean (SD) thickness of type A IMH (n = 48 patients) and type B IMH (n = 103 patients) were 12.7 (6.9) mm (range 5–40 mm) and 10.5 (3.5) mm (range 5–23 mm), respectively. As normal thickness of aorta is less than 3 mm by any imaging modality, we believe that aortic wall thickness ⩾ 5 mm is good enough for diagnosis of IMH in patients with typical clinical symptoms suggesting AAS. The diagnostic sensitivity seems to be not so different among CT, MRI, and TOE, and selection of specific imaging modality depends on many variables including the clinical situation, the physician’s preference, and availability of experts.
Figure 1.
Representative images of aortic intramural haematoma. In pre-contrast computed tomography (A), high attenuation areas along the aortic wall represent intramural haematoma, and no contrast enhancement is characteristic after injection of the contrast agent (B). Magnetic resonance imaging (C) or transoesophageal echocardiography (D) also easily demonstrate crescentic aortic wall thickening without intimal flap or flow communication.
CHALLENGING SCENARIOS
Differential diagnosis
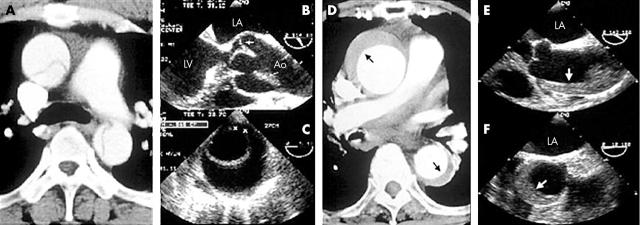
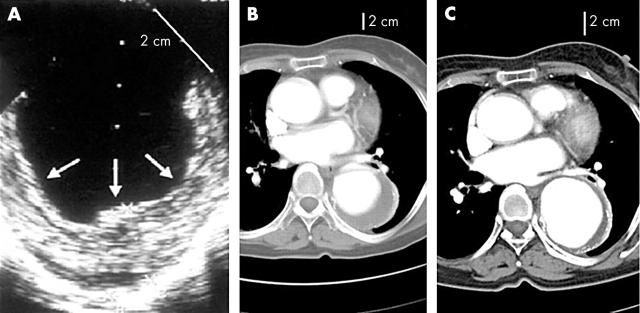
IMH is quite easily differentiated from classic aortic dissection (fig 2); intimal tear or flap is absent and there is no evidence of direct flow communication. As IMH is prevalent in old patients with hypertension, aortic wall thickening caused by atherosclerotic changes or aneurysmal dilatation with mural thrombi, which also occurs frequently in the old, can be a challenging diagnostic problem. In distinguishing IMH from other aortic conditions, identification of the intima and careful observation of the inner surface of the thickened aortic wall is helpful. Usually, the inner margin of IMH is smooth and aortic thickening occurs beneath the bright, echo-dense intima, whereas irregular margin caused by thickening above the intima with dilated aorta is commonly observed in patients with aneurismal dilatation and mural thrombi (fig 3A). In limited patients, IMH can develop at an aortic segment with atherosclerotic changes or aneurysmal dilatation; in this condition, correct diagnosis is more complicated. Fortunately, as these conditions occur predominantly in the descending aorta, follow up imaging study with medical treatment might be helpful; in patients with an IMH component, any changes in the haematoma, either resorption or development of focal ulcer or dissection, are expected, whereas there would be no interval change in atherosclerotic thickening only (fig 3B, C).
Figure 2.
Representative images of classic aortic dissection (A, B, C) and intramural haematoma (D, E, F).
Figure 3.
Images of aortic aneurysm with mural thrombi (A) and aortic intramural haematoma developed at an aortic segment with aneurysmal changes (B, C). In aortic aneurysm with mural thrombi, irregular margin above the intima in a notably dilated aorta is a characteristic finding (A). If aortic intramural haematoma develops in an aortic segment with aneurysmal changes, the inner margin is relatively smooth (B), and usually follow up imaging within several weeks shows changes of aortic thickening, which is quite unusual in mural thrombi. In this case, mural haematoma resolved almost completely with a notable increase of the aortic diameter (C).
Rupture of vasa vasorum versus intimal tear
It is generally believed that IMH is caused by rupture of the vasa vasorum, which separates medial wall layers eventually leading to a secondary tear or to communication to the adventitial space. However, some sporadic case reports showing accidental development of typical IMH by percutaneous catheter manipulations, such as coronary angioplasty,5 insertion of balloon pump,6 or catheter ablation of left sided bypass tract, support the presence of a “primary intimomedial tear” in IMH. This hypothesis is also supported by demonstration of a small intimal communication at the time of surgery.7,8
TOE is superior to any other imaging modality, as it allows direct observation of the aortic intima, and demonstration of flow communication is feasible with the Doppler technique. One characteristic finding of IMH is the presence of “echo-free space” within the thickened aortic wall; the prevalence is higher than 60%,7,9 and some patients with large echo-free space show focal contrast enhancement in CT, which might suggest a small flow communication through the “intimal micro-tear”, which cannot be detected by the conventional echo Doppler technique. In limited patients, contrast injection is helpful for demonstration of direct flow communication through the tear into the echo-free space. The presence or development of echo-free space is not a poor prognostic sign and is not associated with development of classic aortic dissection.9 All these findings suggest the presence of a “micro-tear” in patients with IMH, and the only difference between classic aortic dissection and IMH might be a large intimal tear with a re-entrance tear in aortic dissection. These observations raise the issue of the diagnostic ability of various non-invasive imaging modalities to identify “intimal tear”, which is considered a critical criterion to differentiate classic aortic dissection from IMH. Besides progression from IMH to aortic dissection, some patients exhibit both lesions at the same time, demonstrating a link between them.1 Further investigation is necessary to verify if the “micro-tear” of the large echo-free space is an initiating event of IMH or just a decompression rent.
Prognostic value of diagnostic imaging
The importance of risk stratification based on the findings of diagnostic imaging in patients with AAS cannot be overemphasised. The observations that complete resorption of haematoma is possible with medical treatment, even in type A IMH, and the clinical results of “aggressive” medical treatment are better in type A IMH than in aortic dissection suggest a somewhat positive role for imaging study in the selection of therapeutic strategies.10–13 In both type A and B IMH, haematoma thickness and aortic diameter are reported to be important predictors of adverse clinical events, including death and development of classic aortic dissection.10,14,15
Presenting clinical features are also important predictors of early mortality in patients with AAS. The high prevalence of fluid extravasates, pericardial and pleural effusion, and mediastinal haemorrhage, is a frequent finding in IMH. Among them cardiac tamponade is a great challenge to the attending physician, as mortality is much higher in patients with tamponade and the prevalence of tamponade seems to be higher in patients with type A IMH than in those with aortic dissection. Although successful management of tamponade with pericardiocentesis is sporadically reported,10,11 the safety issue has not been fully tested. Progressive accumulation of large amounts of pleural effusion can occur in patients with an otherwise uncomplicated hospital course, and this finding alone is not an indication of surgical intervention.
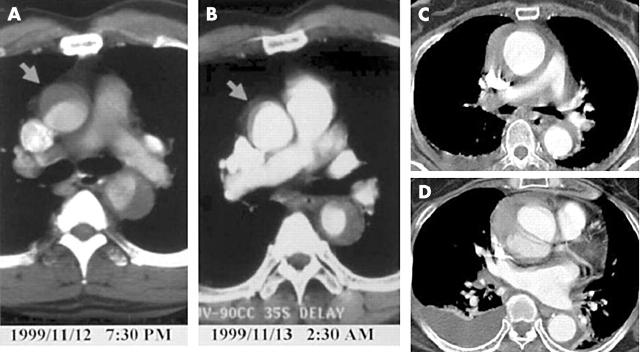
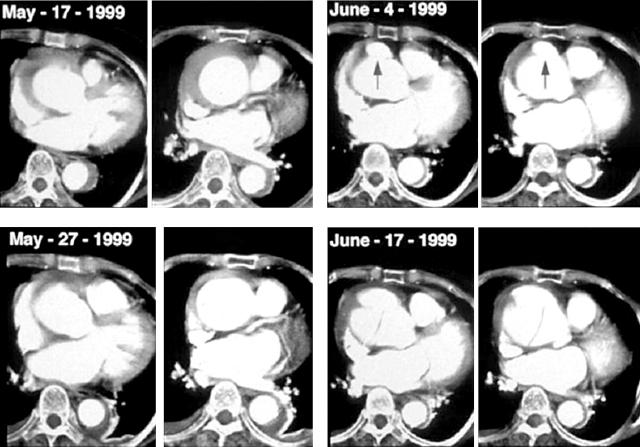
Wall configuration of IMH can change very rapidly, especially in patients with type A IMH; bleeding into the aortic media may be self limiting (fig 4A), but it is also possible to progress to classic aortic dissection or to rupture (fig 4B). Thus, in patients with type A IMH, careful follow up imaging study during the acute phase is absolutely necessary, especially with medical treatment. Development of ulcer-like projection at follow up imaging study is an excellent predictor of progression to classic aortic dissection, especially in type A IMH, and close monitoring is warranted (fig 5).
Figure 4.
Two cases of aortic intramural haematoma showing very rapid changes of aortic wall configuration at the hyper acute stage. The case presented in panels A and B shows a dramatic decrease of the haematoma within seven hours, whereas the case presented in panels C and D shows development of typical aortic dissection within 12 hours after pain onset.
Figure 5.
Ulcer-like projection as a precursor of development of aortic dissection. In this 77 year old woman, after initial improvement of the proximal haematoma with medical treatment, focal ulcer-like projection developed during follow up (June 4 1999). Within two weeks, the patient complained of severe chest pain.
CONCLUSIONS
As the population increasingly ages and survival is prolonged despite hypertension, the incidence of IMH will increase significantly. The availability of modern imaging modalities for aortic pathology enables the attending physicians to investigate the natural history and distinctive clinical features of this disease entity. Besides correct differential diagnosis, imaging studies are expected to provide important prognostic predictors, especially for type A AAS, and this information would be useful for risk stratification or selection of therapeutic strategies along with other clinical variables.
Abbreviations
AAS, acute aortic syndrome
CT, computed tomography
IMH, intramural haematoma
MRI, magnetic resonance imaging
TOE, transoesophageal echocardiography
REFERENCES
- 1.Vilacosta I. Acute aortic syndrome. Heart 2001;85:365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal RC, Chandrasekaran K, Ayala K, et al. Frequency and explanation of false negative diagnosis of aortic dissection by aortography and transesophageal echocardiography. J Am Coll Cardiol 1995;25:1393–401. [DOI] [PubMed] [Google Scholar]
- 3.Yamada T, Tada S, Harada J. Aortic dissection without intimal rupture: diagnosis with MR imaging and CT. Radiology 1988;168:347–52. [DOI] [PubMed] [Google Scholar]
- 4.Mohr-Kahaly S, Erbel R, Kearney P, et al. Aortic intramural hemorrhage visualized by transesophageal echocardiography: findings and prognostic implications. J Am Coll Cardiol 1994;23:658–64. [DOI] [PubMed] [Google Scholar]
- 5.Vilacosta I, Castillo JA, Peral V, et al. Intramural aortic hematoma following intra-aortic balloon counter pulsation. Documentation by transesophageal echocardiography. Eur Heart J 1995;16:2015–6. [DOI] [PubMed] [Google Scholar]
- 6.Vilacosta I, de Dios RM, Pinto AG. Aortic intramural hematoma during coronary angioplasty: insights into the pathogenesis of intramedial hemorrhage. J Am Soc Echocardiogr 2000;13:403–6. [DOI] [PubMed] [Google Scholar]
- 7.Harris KM, Braverman AC, Guitierrez FR, et al. Transesophageal echocardiographic and clinical features of aortic intramural hematoma. J Thorac Cardiovasc Surg 1997;114:619–26. [DOI] [PubMed] [Google Scholar]
- 8.Berdat PA, Carrel T. Aortic dissection limited to the ascending aorta mimicking intramural hematoma. Eur J Cardio Thoracic Surg 1999;15:108–9. [DOI] [PubMed] [Google Scholar]
- 9.Song JM, Kang DH, Song JK, et al. Clinical significance of echo-free space detected by transesophageal echocardiography in patients with type B aortic intramural hematoma. Am J Cardiol 2002;89:548–51. [DOI] [PubMed] [Google Scholar]
- 10.Kaji S, Nishigami K, Akasaka T, et al. Prediction of progression or regression of type A aortic intramural hematoma by computed tomography. Circulation 1999;100(suppl II):II281–6. [DOI] [PubMed] [Google Scholar]
- 11.Song JK, Kim HS, Kang DH, et al. Different clinical features of aortic intramural hematoma versus dissection involving the ascending aorta. J Am Coll Cardiol 2001;37:1604–10. [DOI] [PubMed] [Google Scholar]
- 12.Song JK, Kim HS, Song JM, et al. Outcomes of medically treated patients with aortic intramural hematoma. Am J Med 2002;113:181–7. [DOI] [PubMed] [Google Scholar]
- 13.Sohn DW, Jung JW, Oh BH, et al. Should ascending aortic intramural hematoma be treated surgically? Am J Cardiol 2001;87:1024–6. [DOI] [PubMed] [Google Scholar]
- 14.Sueyoshi E, Imada T, Sakamoto I, et al. Analysis of predictive factors for progression of type B aortic intramural hematoma by computed tomography. J Vasc Surg 2002;35:1179–83. [DOI] [PubMed] [Google Scholar]
- 15.Song JM, Kim HS, Song JK, et al. Usefulness of the initial noninvasive imaging study to predict the adverse outcomes in the medical treatment of acute type A aortic intramural hematoma. Circulation 2003;108(suppl II):II324–8. [DOI] [PubMed] [Google Scholar]