Abstract
A hallmark of calcium-triggered synaptic transmission is the cooperative relationship between calcium and the amount of transmitter released. This relationship is thought to be important for improving the efficiency of synaptic vesicle exocytosis. Although it is generally held that cooperativity arises from the interaction of multiple calcium ions with a single calcium-sensing molecule, the precise molecular basis of this phenomenon is not known. The SNARE proteins are known to be critical for synaptic vesicle exocytosis. We therefore tested for a contribution of SNARE proteins to cooperativity by genetically reducing the levels of syntaxin IA and neuronal-synaptobrevin in Drosophila. Surprisingly, we found that reducing these SNARE proteins also reduced Ca2+ cooperativity. Thus, SNARE proteins are important for determining the cooperative relationship between calcium and synaptic transmission.
Dodge and Rahamimoff (1) first described the phenomenon of calcium cooperativity of synaptic transmission for frog neuromuscular junctions, reporting that the amplitude of excitatory junctional potentials is related to the fourth power of extracellular Ca2+. These observations have been replicated in synapses from many species (2–4) with most having power coefficients of 3–4, suggesting that this is a fundamental property of synaptic transmission. Based on models of cooperative ligand-receptor interactions, these data have been taken to indicate the cooperative involvement of four Ca2+ ions with a Ca2+-sensing molecule in the release of a synaptic vesicle.
Synaptotagmin I (Syt) is a synaptic vesicle protein that is the leading candidate for the Ca2+-sensing molecule regulating synaptic vesicle fusion. It binds multiple Ca2+ ions and binds phospholipids and proteins, including syntaxin, SNAP-25 (synaptosomal-associated protein of 25 kDa), and the assembled SNARE (soluble NSF attachment protein receptor, where NSF is N-ethylmaleimide sensitive fusion protein) complex, in a Ca2+-dependent manner (5, 6). Biophysical analysis of Syt shows that it possesses the properties of a low-affinity Ca2+ sensor (5), and genetic analysis shows that Syt I is clearly required for excitation–secretion coupling (7, 8).
Despite its proposed role in Ca2+ sensing, the role of Syt in conferring cooperativity to synaptic transmission is not well established. Binding of Ca2+ to Syt does not appear to be cooperative, as the second Ca2+ binding site of the Syt C2A domain is estimated to have lower Ca2+ affinity (Kd = ≈400 μM) than the first site (Kd = ≈60 μM) (9). Syt does bind phospholipids in a cooperative Ca2+-dependent manner, but, for this interaction to confer cooperativity to synaptic transmission, it would have to actually cause vesicle fusion. However, Ca2+-triggered synaptic transmission persists in SytI knockout mice (10) although it is severely disrupted. Furthermore, null alleles of Drosophila sytI exhibit Ca2+-triggered synaptic transmission in which Ca2+ cooperativity is not altered (7). These data suggest that the role of Syt is regulatory and not fusogenic. Some Drosophila mutants affecting the C2B domain of Syt are reported to reduce cooperativity (11), but this has been alternatively explained as a change in the Ca2+-dependency curve (12) or it could potentially occur through effects on recycling (13, 14). Finally, reduction of cooperativity has been reported in other variants of Drosophila (e.g., dunce, ref. 15), but it is unlikely the dunce gene product, a phosphodiesterase, is mechanistically involved in Ca2+ cooperativity. Therefore, the precise molecular mechanisms that govern Ca2+ cooperativity of synaptic transmission are unknown.
Other synaptic proteins therefore may be important in determining Ca2+ cooperativity. The SNARE proteins [vesicle-associated membrane protein (VAMP)/synaptobrevin, SNAP-25, and syntaxin] play a fundamental role in synaptic vesicle exocytosis (16). Here, we have examined the contribution of SNARE proteins to Ca2+ cooperativity. To directly test this, we have used Drosophila genetics to independently reduce the level of syntaxin 1A (Syx) or neuronal-synaptobrevin (n-Syb) expression. Surprisingly, we found that reduced levels of these SNARE proteins reduced Ca2+ cooperativity of neurotransmitter release. We discuss this data with respect to molecular models that could account for the role of SNARE proteins in Ca2+ cooperativity of synaptic transmission.
Materials and Methods
Drosophila Stocks.
OregonR (OreR) was the wild-type strain used. n-sybΔF33B is a null allele of the n-syb gene, and n-sybI4 and n-sybI18 are hypomorphic alleles (17). syxΔ229 is a null allele of the syx gene (8), and syxL2 and syxL247 are previously unpublished 5′ P-element insertion alleles originating from the screen described in ref. 18. TM3 and TM6 are balancer chromosomes used to maintain the mutant alleles and are wild type for n-syb and syx. The mutant stocks were maintained as yw; mutant/TM3, Sb Ser, y+ (or TM6, Ubx, y+). Larvae with two mutant chromosomes were identified as yellow larvae emerging from a cross of two mutant lines. Flies with two mutant chromosomes survive to adulthood although their numbers are less than expected, indicating they are semilethal, and the escaping flies are developmentally delayed. Larval movement is sluggish, although they can crawl on the walls of their vials and respond to tactile stimulation.
Protein Analysis.
Protein extracts from adult heads of each genotype were obtained by decapitating the flies under CO2 anesthesia, freezing the heads in tubes maintained on dry ice, manually crushing the heads with a pestle, and resuspending the powder in 50 μl of 1% SDS buffer. The preparations were then centrifuged at 2,000 rpm for 2 min to pellet the cuticle, and equal amounts of protein were separated on 10% SDS/PAGE gels.
Antibodies directed against Drosophila n-Syb (gift of D. Deitcher, Cornell Univ., Ithaca, NY, 1:1,000), syntaxin (mAb 8C3; 1:1,000), and synaptotagmin (dSyt2, gift of H. Bellen, Baylor College of Medicine, Houston; 1:5,000) were used for Western blot analysis of the proteins. Anti-tubulin (mAb E7 1:10) was used to ensure equal loading of each protein sample. Signals were detected with enhanced chemiluminescence (Amersham Pharmacia). Protein levels were estimated by scanning the autoradiographs and measuring the band intensities with IMAGEQUANT 1.1 (Molecular Dynamics) software.
Electrophysiology and Data Analysis.
Two-electrode voltage clamp procedures and HL3 physiological solution used throughout this study have been described (19). A holding potential of −80 mV was maintained with an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA). Data were digitized at 10 kHz and low passed filtered at 2 kHz for evoked junctional currents (EJCs), or 800–1,000 Hz for miniature EJCs (mEJCs), with PCLAMP7 software (Axon Instruments). Evoked and spontaneous currents were analyzed by using the cursor and fit options of clampfit (Axon Instruments). Only fast-rising miniature events were included; slow-rising events originating in electrically connected cells were not analyzed. mEJC decay time constants were measured by aligning, averaging, and fitting exponential curves with MINIANALYSIS (Synaptosoft, Leonia, NJ).
Ca2+ cooperativity was analyzed from synaptic current amplitudes recorded for each Ca2+ concentration from several muscle cells of different larvae. Cooperativity coefficients were derived by fitting linear regression lines to log-transformed individual data points for Ca2+ concentrations ≤1 mM, and the slopes of the regression lines were statistically compared as described (20). Cooperativity coefficients estimated this way closely match coefficients derived by recording from several Ca2+ concentrations in single cells (4), with both techniques giving coefficients of about 3.5 in wild-type larvae. Other statistical analysis was performed with PRISM3 (Graphpad, San Diego). Unless otherwise noted, data are presented as mean ± SEM throughout the text.
Immunofluorescence.
Synaptotagmin distribution was determined by incubating a 1:1,000 dilution dSyt2 anti-synaptotagmin with dissected third instar neuromuscular junctions followed by a 1:500 dilution of FITC goat anti-rabbit secondary antibody. Images were obtained with a Leica confocal microscope and all were acquired at the same gain.
Results
Reduction of Syx and n-Syb.
To investigate the relationship between Ca2+ cooperativity and SNARE proteins, we genetically reduced the levels of the Drosophila SNARE proteins Syx and n-Syb. We tested several combinations of independently generated hypomorphic and null alleles to find the most severe reduction in protein level that supports viability yet may have a physiological phenotype. The use of heteroallelic combinations also eliminates the potential contribution of second site mutations. For syntaxin we found that a combination of two hypomorphic alleles (syxL2/syxL247) and for n-syb a combination of a hypomorphic and a null allele (n-sybI18/n-sybΔF33B) met these requirements.
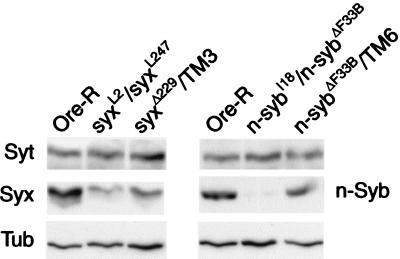
Fig. 1 shows the levels of n-Syb and Syx found by Western blot analysis of protein extracts from adult heads of the indicated genotypes. The combination of syxL2 and syxL247 hypomorphic alleles reduced Syx levels by ≈80%, whereas the combination of null and hypomorphic n-syb alleles (n-sybI18/n-sybΔF33B) reduced n-Syb by ≈90%. We found that n-sybI4 and n-sybI18 had similar effects on protein levels (data not shown), and, in subsequent experiments, data pooled from these two genotypes are referred to as n-sybhypo/n-sybΔF33B. The proteins were reduced by ≈50% in extracts made from null heterozygote heads (n-sybΔF33B/TM6 and syxΔ229/TM3) compared with Ore-R. The same blots were probed with mAb E7 (antitubulin) to demonstrate equal protein loading in each lane.
Figure 1.
Reduction of n-synaptobrevin and syntaxin protein. syxL2/syxL247 and n-sybI18/n-sybΔF33B flies exhibit substantial reduction in protein levels compared with Ore-R controls, whereas Syt levels were unaltered. A lower molecular weight breakdown product of Syt that is recognized by the dSyt2 antibody (46) was similarly unaffected (data not shown). Signals from heterozygote null strains (n-sybΔF33B/TM6 and syxΔ229/TM3) are shown to indicate the 50% reduction level of each protein and anti-tubulin signals are shown to indicate equal protein loading in each lane.
No Change in Synaptotagmin Expression or Synaptic Localization.
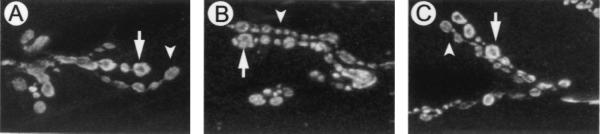
To determine whether the reduction in Syx or n-Syb had altered Syt expression, we reprobed the Western blots with anti-dSyt2 and found no change in the level of Syt protein among the different genotypes (Fig. 1). Previous studies have shown that confocal microscopy is sufficient to detect alterations in Syt subcellular localization when it is perturbed by genetic manipulations (21). We therefore examined the synaptic localization of Syt and found that its distribution appeared unaltered in the mutant genotypes compared with controls. Fig. 2 shows representative Syt staining from neuromuscular junctions of muscle 6 and 7 obtained from control and mutant larvae. These results further show that the innervation of the muscles and morphology of the nerve terminal is apparently not altered in these mutant animals, similar to findings reported for null alleles of these genes (8, 17). Thus any alterations in Ca2+ cooperativity are unlikely to be because of alterations in Syt expression or localization.
Figure 2.
Synaptotagmin distribution is unaltered. Synaptotagmin localization was determined by incubating dissected third-instar neuromuscular junctions from Oregon-R (A), n-sybI4/n-sybΔF33B (B), and syxL2/syxL247 (C) larvae in dSyt2 antisera. The images, representative of four experiments, are taken from the neuromuscular junctions of muscle 6 and 7; the arrows indicate type IB innervation and arrowheads point to type Is innervation demonstrating the presence of the two axons that normally synapse on these muscles. Synaptotagmin localization appears similarly in all genotypes.
Reduced Calcium Cooperativity of Synaptic Transmission.
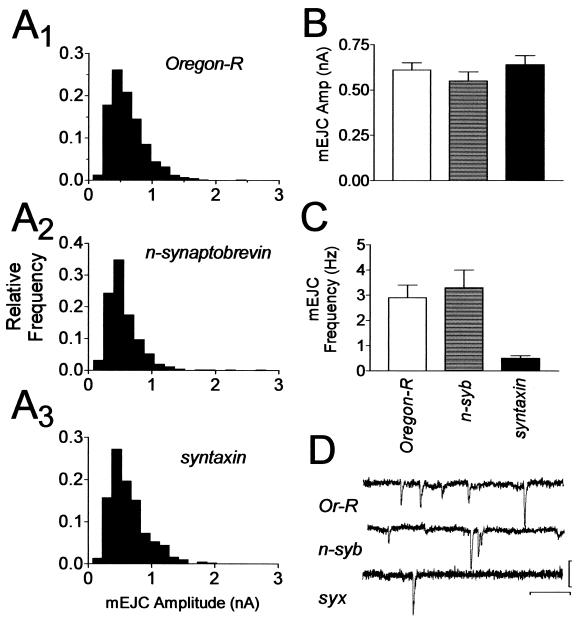
To determine whether the reduction in SNARE protein levels concomitantly altered Ca2+-dependent synaptic transmission, we measured the amplitude synaptic currents (19). We found a reduction of EJCs in both n-sybhypo/n-sybΔF33B and in syxL2/syxL247 larvae over the full range of Ca2+ concentrations tested (Fig. 3 A and B). There was no difference in EC50 values obtained from sigmoidal curves fit to the Ca2+ dose-response data (EC50 values were Ore-R 1.0; n-syb 1.1 and syx 1.1), indicating that sensitivity to calcium was not altered. Furthermore, there were no differences in mEJC amplitude that could explain the observed reduction in synaptic transmission (Fig. 4 A, B, and D). The average mEJC amplitude in all genotypes was about 0.6 nA. We did find an effect on mEJC frequency (Fig. 4C). Ore-R had a mEJC frequency of 2.9 ± 0.5 Hz (n = 6), and n-sybhypo/ n-sybΔF33B had a frequency of 3.3 ± 0.7 Hz (n = 6), whereas syxL2/syxL247 larvae had a lower frequency of 0.5 ± 0.1 Hz (n = 5, P < 0.05, ANOVA).
Figure 3.
Reduced Ca2+ cooperativity in SNARE mutants. (A) Sample EJC traces represent data obtained from Oregon-R (Left), n-sybI4/n-sybΔF33B (Middle), and syxL2/syxL247 (Right) larvae at 0.5 (small trace) and 2.0 mM extracellular Ca2+ (large trace). Calibration: 20 ms, 50 nA. (B) Summary EJC amplitude data plotted on linear coordinates over the entire range of Ca2+ concentrations tested. (C) Summary data for Ca2+ concentrations ≤1 mM plotted on log-log axes. Each point on the graphs in B and C represents the mean EJC amplitude obtained from 6–14 muscle fibers in response to 20–30 stimuli delivered at 0.1 to 1.0 Hz. Symbols: Oregon-R, ■; syxL2/syxL247, ○; n-sybhypo/n-sybΔF33B, ●. Error bars, where visible, are SEM.
Figure 4.
Spontaneous transmitter release in SNARE mutants. (A) Amplitude frequency histograms of 350–400 mEJCs pooled from 3–4 muscle cells for each genotype indicating that the amplitude distributions are not different among the genotypes. (B) Summary of mEJC amplitudes. Each bar is the mean value of the data shown in A. There is no statistical difference between the genotypes (ANOVA, P > 0.05). (C) Summary of mEJC frequency. The data were obtained from 5–6 cells. The frequency observed in syx mutants was significantly lower than in the other two genotypes (ANOVA, P < 0.05). (D) Representative mEJC traces from Oregon-R (upper trace), n-sybI4/n-sybΔF33B (middle trace), and syxL2/syxL247 (lower trace). Calibration: 0.5 nA, 200 ms.
We next estimated Ca2+ cooperativity by analyzing the EJC amplitudes obtained in Ca2+ concentrations of up to 1 mM. Because the EC50 value was not different among the genotypes, we are certain that the responses analyzed were in the same subsaturating region of the Ca2+-dependency curve for each genotype. We estimated Ca2+ cooperativity (n) by measuring the slope of linear regression lines fit to log-transformed individual data points (Fig. 3C). We found that Ore-R larvae had a cooperativity coefficient of 3.4 ± 0.2 (slope ± standard error of the slope), whereas syxL2/syxL247 larvae had a coefficient of 2.6 ± 0.2, and n-sybhypo/n-sybnull larvae had a coefficient of 2.4 ± 0.2. The differences between the slopes of the control and each of the mutants are statistically significant (P < 0.01, ANCOVA and Tukey multiple comparison test) but the slopes of the two mutants are not different from each other. Therefore, reducing the level of SNARE proteins causes a reduction in the Ca2+ cooperativity of synaptic transmission.
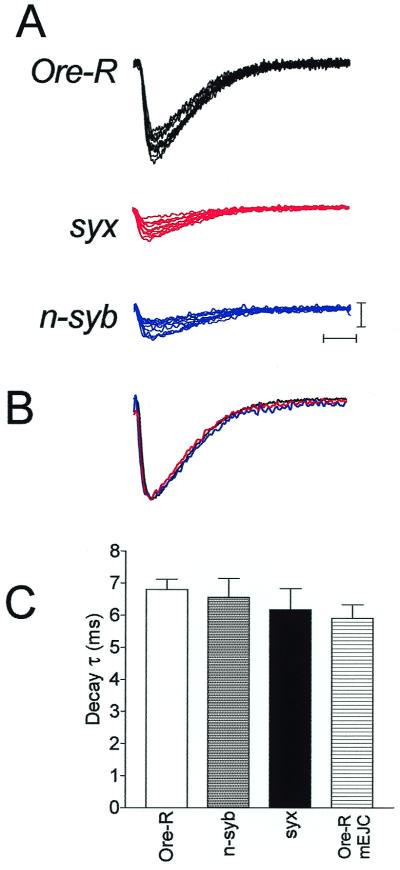
Because the SNARE proteins are known to have biochemical interactions with Ca2+ channels, and have been implicated in localizing synaptic vesicles to the channels, we analyzed whether a change in this spatial arrangement was a factor in the present experiments. If this were important, we would predict that, in addition to shifting the EC50 of the Ca2+ dose-response relationship, individual synaptic responses would have variable latency and would be asynchronous in the mutants. Fig. 5A shows 10 consecutive responses recorded at 0.5 mM Ca2+ from the indicated genotypes and indicates that the onset of the synaptic currents is similar in the mutant and control larvae. To determine whether late releases were more prominent in the mutants than the controls, we averaged and normalized the traces of Fig. 5A and found that the currents overlap in their onset and decay time courses (Fig. 5B). This suggests that late release in the mutant larvae was not more prominent than in controls. To quantify this, decay time constants were measured from the raw data of 5–6 cells at 0.5 mM Ca2+ and revealed no differences among the genotypes (Fig. 5C). These time constants were slightly, but not significantly (P > 0.6, ANOVA), longer than the decay time constant of spontaneous mEJCs in Ore-R preparations.
Figure 5.
Synchronicity of synaptic transmission in SNARE mutants. (A) Ten consecutive responses recorded at 0.5 mM extracellular Ca2+ from the indicated genotypes demonstrating the synchronous onset of the synaptic current in each. The stimulus artifact has been removed for clarity. Calibration: 5 nA, 5 ms. (B) Averaged and normalized traces derived from those in A demonstrate the overlapping onset and decay time courses of the synaptic current from the different genotypes. (C) Synaptic current decay time constants were measured from 5–6 cells, each the mean response of 20 stimuli, for each genotype and averaged. There are no differences among the genotypes. For comparison, the mean mEJC decay time constant from four Ore-R cells, representing 280 mEJCs, is shown.
Discussion
SNARE proteins are known to be important for membrane trafficking and exocytosis. Mutational analysis of these proteins in Drosophila has contributed significantly to our understanding of the mechanistic role SNAREs play in synaptic transmission. Previous studies of syntaxin and n-syb null alleles have shown a differential sensitivity of spontaneous release to elimination of these two proteins. Null alleles of syntaxin abolish spontaneous release, whereas n-syb nulls reduce it by about 75% (8, 17). Our data indicate that this relationship also exists in hypomorphic alleles of these genes, indicating the requirement for syntaxin in some fusion events in which n-Syb is not required. These results may be explained by t-SNARE/t-SNARE mediated fusion, which can occur with yeast endosomes (22) or, alternatively, that some spontaneous fusion events use an alternative isoform of synaptobrevin that cannot substitute for n-synaptobrevin in Ca2+-triggered fusion.
The hypomorphic alleles of Drosophila syntaxin and n-synaptobrevin also allowed us to test for a role of these proteins in Ca2+ cooperativity of synaptic transmission. Our studies revealed that the SNARE proteins make a direct contribution to Ca2+ cooperativity of neurotransmitter release. One potential mechanism by which SNAREs could contribute to cooperativity is if they have a positive influence on Ca2+ channel activity and that the SNARE mutants we used here inhibit Ca2+ influx. However, this seems unlikely for three reasons. First, several studies have shown that synaptic Ca2+ currents are not reduced by treatment with Tetanus or Botulinum neurotoxins (23–27), proteases that cleave SNARE proteins. Second, coexpression studies using mammalian recombinant proteins (28–30) show that syntaxin inhibits, not enhances, Ca2+ channel activity. Lastly, transmitter release is enhanced in Drosophila in which syntaxin binding to the synprint domain of Ca2+ channels is abolished (31), a change opposite to that expected if SNAREs enhance Ca2+ flux. Therefore, in the mutants we used here, it seems unlikely that the change in cooperativity we measured is caused by reduced Ca2+ channel function.
A second potential mechanism could be that, by reducing SNARE proteins, we have altered the normal spatial arrangement of the Ca2+ sensor and the Ca2+ channel. As outlined by Klingauf and Neher (32), this could lead to an apparent change in cooperativity because of nonlinear diffusion of calcium at the release site. However, a change in this spatial arrangement is also predicted to increase the EC50 value of the Ca2+ dose-response curve (33) and to increase the variability of synaptic release times (34). Although such changes are observed in an n-syb null allele (35), with the alleles used presently we did not observe a shift in the EC50 value of our dose-response curves, nor did we see differences in either synaptic current onset or in the current decay time constant. These data indicate that a spatial disruption is not the underlying cause of the change in cooperativity that we observed and if syntaxin or n-synaptobrevin is important for localization of vesicles to Ca2+ channels the mutants we used here appear to produce sufficient protein to perform this function.
Our findings are in accord with earlier studies (36–38) that showed that Ca2+ cooperativity of transmitter release is reduced in Botulinum toxin A (BoTxA, which cleaves SNAP-25) and tetanus toxin (which cleaves VAMP/synaptobrevin) poisoned rat neuromuscular junctions. We report the involvement of syntaxin in Ca2+ cooperativity and altogether these studies show that independent manipulation of any one of the three SNARE proteins can alter cooperativity.
Of particular importance is our observation that cooperativity can be reduced in syx and n-syb mutants without alterations in Ca2+ sensitivity, suggesting that the molecular mechanisms that regulate Ca2+ cooperativity and Ca2+ sensing are distinct. This result differs from BoTxA studies in which inhibition of transmitter release by the toxin can be reversed by increasing intracellular Ca2+ (36, 39). Together these data suggest that whereas a small C-terminal fragment of SNAP-25 may be important for an interaction that determines Ca2+ sensitivity, the full SNARE complex is important for determining Ca2+ cooperativity.
Because syntaxin and n-Syb protein expression is reduced in our mutants, and the SNARE proteins do not appear to have intrinsic Ca2+-sensing activity, it seems that the number of SNARE proteins is a critical determinant of cooperativity. There is increasing evidence to support the view that trans-SNARE complexes exist in a preformed “loose” state and their zippering into a tightly associated complex results in an increased rate of vesicle fusion (40–43). We assume multiple trans-SNARE complexes normally participate in fusion and propose that cooperativity is a consequence of the Ca2+-dependent (44) formation of one “tight” trans-SNARE complex increasing the likelihood of neighboring loose complexes making the transition to tight complexes (Fig. 6).
Figure 6.
Model for Ca2+ cooperativity of synaptic transmission. Under resting conditions, wild-type (WT) flies have the full complement of SNARE proteins and SNARE complexes exist in a preformed “loosely” associated state (green). Upon the admittance of Ca2+, SNARE complexes make the transition from the loose state to a “tight” state (purple). Cooperativity arises when the formation of a tight SNARE complex increases the probability that neighboring loose complexes will form tight complexes in a Ca2+-dependent manner (red). If there is a reduced-level SNARE protein (Mutant) when a complex makes the transition from loose to tight, there are fewer neighboring complexes that can be influenced by it, and thus cooperativity is reduced.
We include in this model the potential that other proteins may regulate SNARE complex zippering in a Ca2+-dependent manner. The key point of our model is that cooperativity is a function of the number of complexes that can participate in fusion. In the mutant studies presented here, independently reducing the level of one of the SNARE proteins reduces the number of complexes and thus reduces cooperativity and this can occur independently of any change in Ca2+ sensitivity. In an alternative model, in which synaptic vesicle exocytosis is normally prevented by a “brake,” cooperativity could arise if the Ca2+-dependent removal of a brake from one SNARE increases the likelihood of remaining brakes being removed. Thus, the red SNARE complexes in Fig. 6 would represent those that have a high probability of losing their brake.
Our hypothesis implies that SNARE complexes can interact and this could potentially occur through protein–protein interactions. Poirier et al. (45) have suggested that SNAP-25 may contribute to SNARE complex oligomerization if its C-terminal α-helix participates in a different SNARE complex than its N-terminal α-helix. Other proteins that interact with the SNARE complex, such as complexin, or Ca2+-dependent oligomerization of synaptotagmin also may mediate this function. In any case, formation of one SNARE complex could induce mechanical strain on the neighboring complexes and yield vesicles that are more responsive to Ca2+. Experiments designed to explore this hypothesis will provide valuable insights into the molecular mechanisms that control calcium cooperativity of synaptic transmission.
Acknowledgments
We thank Drs. T. Schwarz, D. Deitcher, H. Bellen, and the Developmental Studies Hybridoma Bank (University of Iowa) for the provision of fly stocks and antibodies; Dr. P. Dufort for help with statistical analysis; and Dr. L. Y. Wang for helpful discussions. This work was supported by grants from Medical Research Council Canada (G.L.B.) and Natural Sciences and Engineering Research Council (W.S.T.). B.A.S. is the recipient of a Fellowship from The Hospital for Sick Children Research Training Center.
Abbreviations
- Syt
synaptotagmin I
- SNAP-25
synaptosomal-associated protein of 25 kDa
- VAMP
vesicle-associated membrane protein
- Syx
syntaxin 1A
- n-Syb
neuronal synaptobrevin
- EJC
evoked junctional current
- mEJC
miniature EJC
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250491397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250491397
References
- 1.Dodge F A, Jr, Rahamimoff R. J Physiol (London) 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith S J, Augustine G J, Charlton M P. Proc Natl Acad Sci USA. 1985;82:622–625. doi: 10.1073/pnas.82.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidelberger R, Heinemann C, Neher E, Matthews G. Nature (London) 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- 4.Jan L Y, Jan Y N. J Physiol (London) 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis A F, Bai J, Fasshauer D, Wolowick M J, Lewis J L, Chapman E R. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 6.Sudhof T C, Rizo J. Neuron. 1996;17:379–388. doi: 10.1016/s0896-6273(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 7.Broadie K, Bellen H J, DiAntonio A, Littleton J T, Schwarz T L. Proc Natl Acad Sci USA. 1994;91:10727–10731. doi: 10.1073/pnas.91.22.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze K L, Broadie K, Perin M S, Bellen H J. Cell. 1995;80:311–320. doi: 10.1016/0092-8674(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 9.Shao X, Davletov B A, Sutton R B, Sudhof T C, Rizo J. Science. 1996;273:248–251. doi: 10.1126/science.273.5272.248. [DOI] [PubMed] [Google Scholar]
- 10.Geppert M, Goda Y, Hammer R E, Li C, Rosahl T W, Stevens C F, Sudhof T C. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 11.Littleton J T, Stern M, Perin M, Bellen H J. Proc Natl Acad Sci USA. 1994;91:10888–10892. doi: 10.1073/pnas.91.23.10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parfitt K, Reist N, Li J, Burgess R, Deitcher D, DiAntonio A, Schwarz T L. Cold Spring Harbor Symp Quant Biol. 1995;60:371–377. doi: 10.1101/sqb.1995.060.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J Z, Davletov B A, Sudhof T C, Anderson R G. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen E M, Hartwieg E, Schuske K, Nonet M L, Jin Y, Horvitz H R. Nature (London) 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- 15.Zhong Y, Wu C F. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- 16.Bock J B, Scheller R H. Proc Natl Acad Sci USA. 1999;96:12227–12229. doi: 10.1073/pnas.96.22.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deitcher D L, Ueda A, Stewart B A, Burgess R W, Kidokoro Y, Schwarz T L. J Neurosci. 1998;18:2028–2039. doi: 10.1523/JNEUROSCI.18-06-02028.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess R W, Deitcher D L, Schwarz T L. J Cell Biol. 1997;138:861–875. doi: 10.1083/jcb.138.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart B A, Atwood H L, Renger J J, Wang J, Wu C F. J Comp Physiol A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 20.Zar J. Biostatistical Analysis. Englewood Cliffs, NJ: Prentice Hall; 1984. [Google Scholar]
- 21.Fergestad T, Davis W S, Broadie K. J Neurosci. 1999;19:5847–5860. doi: 10.1523/JNEUROSCI.19-14-05847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols B J, Ungermann C, Pelham H R, Wickner W T, Haas A. Nature (London) 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- 23.Dreyer F, Mallart A, Brigant J L. Brain Res. 1983;270:373–375. doi: 10.1016/0006-8993(83)90617-0. [DOI] [PubMed] [Google Scholar]
- 24.Mochida S, Saisu H, Kobayashi H, Abe T. Neuroscience. 1995;65:905–915. doi: 10.1016/0306-4522(94)00508-3. [DOI] [PubMed] [Google Scholar]
- 25.Marsal J, Ruiz-Montasell B, Blasi J, Moreira J E, Contreras D, Sugimori M, Llinas R. Proc Natl Acad Sci USA. 1997;94:14871–14876. doi: 10.1073/pnas.94.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Llinas R, Sugimori M, Chu D, Morita M, Blasi J, Herreros J, Jahn R, Marsal J. J Physiol (London) 1994;477:129–133. doi: 10.1113/jphysiol.1994.sp020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molgo J, Siegel L S, Tabti N, Thesleff S. J Physiol (London) 1989;411:195–205. doi: 10.1113/jphysiol.1989.sp017568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiser O, Trus M, Hernandez A, Renstrom E, Barg S, Rorsman P, Atlas D. Proc Natl Acad Sci USA. 1999;96:248–253. doi: 10.1073/pnas.96.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergsman J B, Tsien R W. J Neurosci. 2000;20:4368–4378. doi: 10.1523/JNEUROSCI.20-12-04368.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bezprozvanny I, Scheller R H, Tsien R W. Nature (London) 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 31.Wu M N, Fergestad T, Lloyd T E, He Y, Broadie K, Bellen H J. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- 32.Klingauf J, Neher E. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rettig J, Heinemann C, Ashery U, Sheng Z H, Yokoyama C T, Catterall W A, Neher E. J Neurosci. 1997;17:6647–6656. doi: 10.1523/JNEUROSCI.17-17-06647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mochida S, Sheng Z H, Baker C, Kobayashi H, Catterall W A. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 35.Yoshihara M, Ueda A, Zhang D, Deitcher D L, Schwarz T L, Kidokoro Y. J Neurosci. 1999;19:2432–2441. doi: 10.1523/JNEUROSCI.19-07-02432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cull-Candy S G, Lundh H, Thesleff S. J Physiol (London) 1976;260:177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bevan S, Wendon L M. J Physiol (London) 1984;348:1–17. doi: 10.1113/jphysiol.1984.sp015095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson L L. J Pharmacol Exp Ther. 1978;206:661–669. [PubMed] [Google Scholar]
- 39.Lundh H, Leander S, Thesleff S. J Neurol Sci. 1977;32:29–43. doi: 10.1016/0022-510x(77)90037-5. [DOI] [PubMed] [Google Scholar]
- 40.Hua S Y, Charlton M P. Nat Neurosci. 1999;2:1078–1083. doi: 10.1038/16005. [DOI] [PubMed] [Google Scholar]
- 41.Lin R C, Scheller R H. Neuron. 1997;19:1087–1094. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 42.Scales S J, Chen Y A, Yoo B Y, Patel S M, Doung Y C, Scheller R H. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 43.Xu T, Rammner B, Margittai M, Artalejo A R, Neher E, Jahn R. Cell. 1999;99:713–722. doi: 10.1016/s0092-8674(00)81669-4. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y A, Scales S J, Patel S M, Doung Y C, Scheller R H. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 45.Poirier M A, Hao J C, Malkus P N, Chan C, Moore M F, King D S, Bennett M K. J Biol Chem. 1998;273:11370–11377. doi: 10.1074/jbc.273.18.11370. [DOI] [PubMed] [Google Scholar]
- 46.Littleton J T, Bellen H J, Perin M S. Development (Cambridge, UK) 1993;118:1077–1088. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]