Abstract
Clinical data are reported for 13 patients who were referred with recurrent loss of consciousness at night interrupting their sleep. Most of the patients were women (10 of 13) with a mean age of 45 years (range 21–72 years). The histories were more consistent with vasovagal syncope than with epilepsy. This was supported by electroencephalographic and tilt test results. More polysomnographic monitoring data are required to confirm the diagnosis of vasovagal syncope interrupting sleep. This will be difficult because, although the condition may not be rare, the episodes are usually sporadic.
Keywords: epilepsy, sleep, vasovagal syncope
When a patient complains of episodic loss of consciousness at night interrupting sleep, most physicians think of epilepsy as the likely diagnosis. The differential diagnosis includes sleep apnoea, sleep paralysis, hypoglycaemia, panic attacks, and cardiac arrhythmia.1 What is not usually considered is an unusual presentation of vasovagal syncope, the most common cause of transient loss of consciousness.2 Because of current teaching, physicians are reluctant to diagnose a vasovagal mechanism when syncope occurs in a supine patient.1 However, such reactions have been frequently observed during venesection, surgical procedures, and cardiac catheterisation.3 Furthermore, transient autonomic mechanisms that predispose to vasovagal syncope may occur during sleep.4 We present a series of 13 patients with recurrent episodes of nocturnal syncope. Detailed histories were taken in an effort to ascertain the likely cause of these episodes, in particular whether the clinical features were suggestive of a vasovagal reaction starting during sleep.
PATIENT HISTORIES
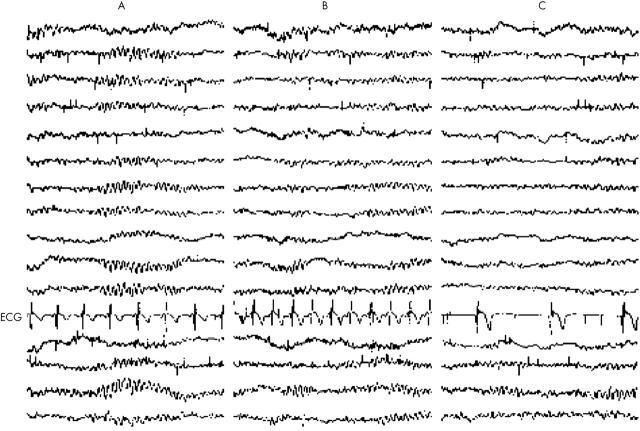
Patient 1 had her first nocturnal syncopal episode at the age of 40 years. After sleeping for some hours, she awoke and felt nauseous with abdominal discomfort and the urge to defecate. She lost consciousness while lying supine. She sweated profusely but did not bite her tongue. Her husband observed transient myoclonic jerking. After this, similar episodes occurred regularly (at least one a month) and only at night. The syncopal episodes never exceeded one minute and were atraumatic. She was incontinent of urine and faeces once. A tilt test provoked a vasovagal reaction followed by seven seconds of asystole and reproduced her nocturnal symptoms. Because of ongoing symptoms she underwent neurological investigations and a typical nocturnal episode was recorded during continuous electroencephalographic (EEG) and cardiographic monitoring (fig 1). The EEG was judged normal by two independent neurologists. The ECG, however, showed a pronounced bradycardia (36 beats/min) during the episode, with an atrioventricular node escape rhythm.
Figure 1.
Simultaneous electroencephalographic (EEG) and cardiac recordings before and during typical nocturnal episode in patient 1. The montage consists of four sets of channels running anteriorly to posteriorly and recorded from the right parasagittal, left parasagittal, right temporal, and left temporal areas. (A) Time 5.49 am, normal sleep EEG, heart rate 90 beats/min. (B) Time 5.50 am, EEG unchanged, heart rate 126 beats/min. (C) Time 5.52 am (after calling for a nurse), with the patient lying supine in bed unable to move, EEG unchanged, heart rate 36 beats/min. At this time the patient was pale and sweating profusely.
At three syncope units worldwide we saw 13 patients with nocturnal episodes similar to those that patient 1 was having (table 1). Ten were women. The mean age was 45 years (range 21–72 years). They all gave a history of waking up at night with nausea and urge to defecate. In some patients, syncope occurred in bed; in others immediately after leaving the bed in an effort to get to the toilet. The syncope was of short duration and was often accompanied by profuse sweating. After regaining consciousness most patients felt very weak and could not remain upright but were orientated. Bradycardia was documented in five patients. The frequency of attacks varied from weekly to annually and there was no relation to menstruation or alcohol. Three patients reported nightmares immediately before the episode. Some patients had learned to partially abort the episodes by remaining supine in bed. Nine patients also had daytime syncopal and presyncopal episodes associated with vasovagal symptoms.
Table 1.
Patient demographic and clinical data
| Patient | Age (years) | Sex | Total noct | Total past year | Daytime VVS | Symptoms during LOC | Body position | Duration LOC (s) | Symptoms after LOC | Tilt | EEG | Comments | ||||
| Nausea | Abdo | Defec | Alert | Weak | Tired | |||||||||||
| 1 | 48 | F | 100 | 15 | No | Yes | Yes | Yes | Horiz | 60 | Yes | Yes | Yes | +ve | Normal | Bradycardia |
| 2 | 38 | F | 20 | 4 | No | Yes | Yes | Yes | Vertical | 60 | Yes | Yes | No | −ve | N/D | Clusters |
| 3 | 65 | F | 10 | 2 | Yes | Yes | Yes | Yes | Vertical | 120 | Yes | Yes | Yes | +ve | Normal | Clusters |
| 4 | 50 | F | 5 | 2 | Yes | Yes | No | Yes | Horiz | 30 | Yes | No | Yes | +ve | Normal | None |
| 5 | 72 | F | 8 | 2 | Yes | Yes | Yes | Yes | Vertical | 60 | Yes | Yes | Yes | −ve | N/D | Bradycardia |
| 6 | 41 | F | 5 | 1 | No | Yes | Yes | Yes | Horiz | 5 | Yes | Yes | Yes | N/D | N/D | Bradycardia |
| 7 | 21 | F | 20 | 10 | No | Yes | Yes | Yes | Vertical | 30 | Yes | No | Yes | +ve | Normal | None |
| 8 | 64 | M | 75 | 10 | Yes | No | Yes | Yes | Horiz | 1200 | Yes | Yes | Yes | N/D | N/D | Bradycardia |
| 9 | 39 | M | 9 | 9 | Yes | Yes | No | Yes | Vertical | 60 | Yes | Yes | Yes | −ve | N/D | None |
| 10 | 70 | M | 4 | 2 | Yes | Yes | Yes | No | Horiz | 60 | Yes | Yes | Yes | +ve | N/D | Bradycardia |
| 11 | 24 | F | 20 | 4 | Yes | Yes | Yes | Yes | Horiz | 180 | Yes | Yes | No | +ve | Abnormal | Clusters |
| 12 | 30 | F | 5 | 3 | No | Yes | Yes | Yes | Horiz | 180 | Yes | No | Yes | +ve | Normal | None |
| 13 | 28 | F | 5 | 3 | No | Yes | Yes | Yes | Horiz | 180 | Yes | No | No | −ve | Normal | None |
+ve, syncope during 30 minutes of head-up 60° tilt; −ve, no syncope during tilt; Abdo, abdominal pain; Bradycardia, heart rate < 50 beats/min during nocturnal episode; Clusters, episodes clustered in time; Defec, urge to defecate; Duration LOC, maximum time spent unconscious during an episode; EEG, interictal electroencephalogram; F, female; Horiz, horizontal body position at time of attack; M, male; N/D, not done; noct, total number of episodes interrupting sleep during lifetime; Symptoms after LOC, symptoms during recovery after loss of consciousness; Symptoms during LOC, symptoms immediately before or after loss of consciousness; Tilt, head-up tilt test result; Total past year, total number of episodes interrupting sleep during past year; VVS, vasovagal syncope episodes.
Eleven patients underwent tilt table testing (without pharmacological provocation). The test was positive in seven with typical prodromal symptoms. A significant (asystole > 3 s) cardioinhibitory reaction was recorded in four of the seven. The possibility of organic cardiac or cerebral pathology as a cause of the episodes was excluded by appropriate additional testing. Interictal EEG performed in seven patients showed epileptiform activity in one (patient 11).
DISCUSSION
We suggest that all of these patients may have nocturnal vasovagal syncope as the primary cause of their symptoms. Although the attacks started when the patients were supine, the associated symptoms described were typical of vasovagal syncope. These were nausea, sweating, lightheadedness, and abdominal discomfort during the attack, followed by tiredness and weakness afterwards. These symptoms were reproduced by tilt testing in 64% of the group. Because the attacks occurred at night in bed, epilepsy was often initially diagnosed, especially if muscle jerking was observed. However, it should be realised that transient myoclonic jerking is more often a feature of cerebral hypoperfusion than of epilepsy.2 Other more reliable features of epilepsy, including tongue biting, automatisms, postictal confusion, and hypersomnolence, were absent.5 On the basis of an algorithm derived from a recent study on historical criteria that distinguish syncope from seizures, all of our patients fulfilled the diagnostic criteria for vasovagal syncope with very high levels of certainty (> 90%).6 Furthermore, we obtained a normal EEG during a typical nocturnal episode in one patient and normal interictal EEGs in 46% of the group. In addition to the nocturnal episodes, most of these patients had daytime vasovagal attacks in response to common triggers—for example, pain, the sight of blood, and prolonged standing in hot, crowded rooms.
It is most important to differentiate nocturnal syncope from atypical forms of epilepsy. For example, abdominal epilepsy is a rare form of complex partial seizure disorder affecting women of a similar age to those of our patients.7 In a series of 10 patients, most had protracted abdominal pain with headaches and confusion but not nocturnal loss of consciousness. Other types of complex partial seizures begin with transient upper abdominal discomfort and may be complicated by autonomic symptoms.1 Nocturnal epilepsy, associated with prolonged gastrointestinal and autonomic symptoms, is seen in childhood but typical seizure manifestations are also present.8 Complex partial seizures in most patients are diagnosed by the presence of spike and slow wave complexes on the interictal EEG. This paroxysmal activity may be more often seen during sleep.1,5,7 However, in patients with vasovagal syncope, the interictal EEG is normal.5 Even during syncopal reactions induced in the laboratory, only large amplitude slow waves are seen and there is no paroxysmal activity.9 As blood pressure falls and consciousness is lost, diffuse flattening of the waveforms occurs. This EEG pattern is thought to be reactive and represents a generalised “slowing down” of nervous activity in response to severe hypotension and hypoperfusion of the brain.
The mechanism for vasovagal syncope remains uncertain but probably involves transient inhibition of sympathetic outflow from the medulla in response to certain triggers such as orthostasis and fear.3 We are postulating that this reaction can occur during supine sleep when, certainly, the orthostatic trigger does not apply. But there are several other possible mechanisms by which the brainstem may be transiently overridden: firstly, asymmetrical slow wave activity in the cortex10; secondly, rapid changes in sympathetic activity occurring during the transition between the stages of sleep11; thirdly, cortical inhibitory discharge as the result of a nightmare4; and lastly, gastrointestinal afferents firing paradoxically in response to visceral stimuli, as in defecation syncope.12 We consider that the final possibility is unlikely to occur and that abdominal discomfort is more likely to be an effect rather than a cause of increased vasovagal activity in our patients.13 There are several mechanisms by which major changes in visceral autonomic tone may cause abdominal pain.14
Finally, we emphasise that our findings are only preliminary and our data are incomplete. The diagnosis of episodic nocturnal conditions is difficult because the doctor does not usually ever see what happens during an event. Taking an expert history and excluding other possibilities is a good start but does not prove the diagnosis. On the basis of our observations and limited data, we think that patients who have nocturnal loss of consciousness with classical vasovagal prodromal symptoms may have true vasovagal syncope. A history of daytime vasovagal syncope in response to typical triggers and a positive tilt table test can support this diagnosis. Most cardiac causes of nocturnal syncope, including repolarisation abnormalities, can be excluded by a normal ECG.2 Interictal (including sleep) EEG is useful to exclude epilepsy, which is the main differential diagnosis, remembering that this test has a sensitivity of only 0.5–0.75 in most series.1,5,15 Ideally, we require video monitoring of further episodes in other patients with continuous polysomnographic recordings. This will be difficult in view of the sporadic nature of the attacks in most patients. We suspect that this condition is not rare, despite the absence of previous reports. Doctors may discount the diagnosis of vasovagal syncope interrupting sleep because the concept does not fit with current physiological dogma. Patients may not volunteer their nocturnal episodes for fear of being labelled epileptic or hysterical. Doctors managing patients with recurrent vasovagal syncope who inquire specifically about nocturnal episodes may be reminded of patients with similar histories to those we have described. The fact that these cases have not been previously reported is probably testimony to our lack of understanding, rather than the true incidence, of this condition.
Acknowledgments
The authors thank J H T M Koelman MD PhD, clinical neurophysiologist, Department of Neurology, Academic Medical Centre/University of Amsterdam, the Netherlands, for his re-evaluation of the EEG. The figure was prepared by the Department of Medical Illustration, Christchurch Hospital.
Source of support: Dr Wieling is the recipient of an unrestricted educational grant from Medtronic Europe.
REFERENCES
- 1.Hopkins AP. Disorders of consciousness. In: Weatherall DJ, Ledlingham JGG, Warrell DA, eds. Oxford textbook of medicine, 3rd ed. Oxford: Oxford University Press, 1996:3909–25.
- 2.Brignole M, Alboni P, Benditt D, et al. Guidelines on management (diagnosis and treatment) of syncope. Eur Heart J 2001;22:1256–306. [DOI] [PubMed] [Google Scholar]
- 3.Van Lieshout JJ, Wieling W, Karemaker M, et al. The vasovagal response. Clin Sci (Lond) 1991;81:575–86. [DOI] [PubMed] [Google Scholar]
- 4.Shneerson JM. Handbook of sleep medicine. Oxford: Blackwell Science, 2000:1–15.
- 5.Lempert T. Recognizing syncope. J R Soc Med 1996;89:372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheldon R, Rose S, Ritchie D, et al. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol 2002;40:142–8. [DOI] [PubMed] [Google Scholar]
- 7.Peppercorn MA, Herzog AG. The spectrum of abdominal epilepsy in adults. Am J Gastroenterol 1989;84:1294–6. [PubMed] [Google Scholar]
- 8.Koutroumanidis M. Panayiotopoulos syndrome. BMJ 2002;324:1228–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lempert T, Bauer M, Schmidt D. Syncope: a videometric analysis of 56 episodes of transient cerebral hypoxia. Ann Neurol 1994;36:233–7. [DOI] [PubMed] [Google Scholar]
- 10.Mercader MA, Varghese PJ, Potolicchio SJ, et al. New insights into the mechanism of neurally mediated syncope. Heart 2002;88:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328:303–7. [DOI] [PubMed] [Google Scholar]
- 12.Pathy MS. Defaecation syncope. Age Aging 1978;7:233–6. [DOI] [PubMed] [Google Scholar]
- 13.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology 2000;118:842–8. [DOI] [PubMed] [Google Scholar]
- 14.Orr WC. Gastrointestinal physiology. In: Kryger MH, Roth T, Dement WC, eds. Principles and practice of sleep medicine, 3rd ed. Philadelphia: WB Saunders, 2000:279–88.
- 15.Hoefnagels WA, Padberg GW, Overweg J, et al. Syncope or seizure? The diagnostic value of the EEG and hyperventilation test in transient loss of consciousness. J Neurol Neurosurg Psychiatry 1991;54:935–6. [DOI] [PMC free article] [PubMed] [Google Scholar]