The bone marrow derived endothelial progenitor cells (EPCs) are considered to originate from haematopoietic stem cells, which are positive for CD34.1 Human CD34 expressing cells (CD34+ cells) injected into nude mice and rats undergoing neovascularisation caused by hindlimb ischaemia are incorporated into the neovasculature and express endothelial antigens. We recently reported that transplantation of autologous bone marrow cells, including CD34+ cells, improved ischaemia in patients with critical limb ischaemia.2 Moreover, increased neovascularisation by bone marrow derived CD34+ cells was shown to improve cardiac function.
Recently, a mobilisation of EPCs into circulation from bone marrow was reported in patients with acute myocardial infarction and acute coronary syndrome,3,4 but little is known about the regulation of EPC mobilisation in patients with stable coronary artery disease (CAD). In the present study, we investigated the number of circulating CD34+ cells in patients with CAD and the influence of atherosclerotic risk factors on this number.
METHODS
Thirty four patients (mean (SEM) age 62.5 (1.7) years) with angiographically documented stable CAD, and 36 healthy control subjects (mean (SEM) age 53.6 (2.0) years) without any evidence of CAD by history and physical examination, were enrolled in the present study. Risk factors for CAD were defined as: a history of hypertension for more than one year that required the initiation of antihypertensive treatment; a history of smoking more than one pack per year and currently smoking; hyperlipidaemia, defined as total cholesterol concentrations exceeding 5.70 mmol/l; and diabetes mellitus, defined as the need for oral anti-diabetic drug treatment or insulin use. The study protocol was approved by the ethics committee of Jichi Medical School, and informed consent was obtained from all patients and control subjects.
The number of CD34+ cells in white blood cells (WBCs) was quantified by FACScan (Becton-Dickinson). In brief, WBCs were stained with a fluorescein isothiocyanate conjugated anti-CD34 monoclonal antibody (Becton-Dickinson, Franklin Lakes, New Jersey, USA). The samples were subjected to a two dimensional side scatter fluorescence dot plot analysis. After appropriate gating, the number of CD34+ cells with low cytoplasmic granularity (low sideward scatter) was quantified and expressed as the number of cells per 103 WBCs.
Values were expressed as mean (SEM). Significance was evaluated using unpaired Student’s t test for comparisons between two means. The interaction between the number of CD34+ cells and risk factors was examined by multivariate analysis using the multiple stepwise logistic regression model. Differences of p < 0.05 were considered significant.
RESULTS
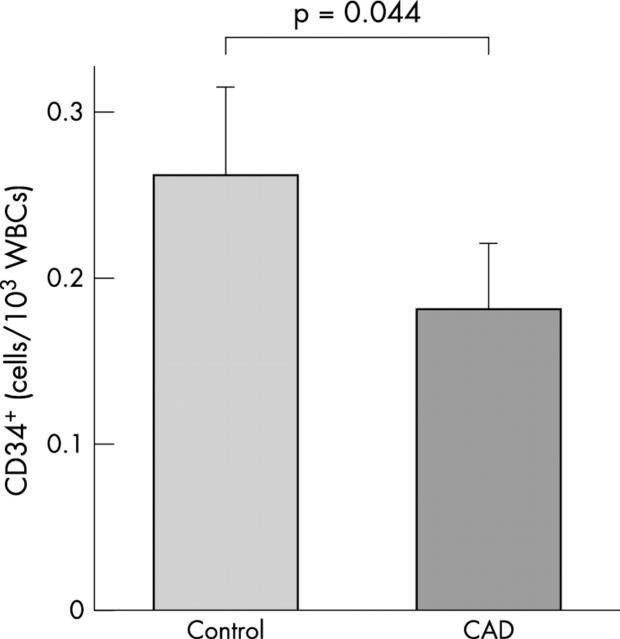
The number of CD34+ cells in the peripheral blood of patients with CAD and control subjects was determined by flow cytometry. As shown in fig 1, circulating CD34+ cells were significantly reduced, by approximately 30%, in patients with CAD compared with age matched control subjects.
Figure 1.
The number of circulating CD34+ cells. CD34+ cells were detected in peripheral blood from patients with CAD (n = 34) and healthy control subjects (n = 36).
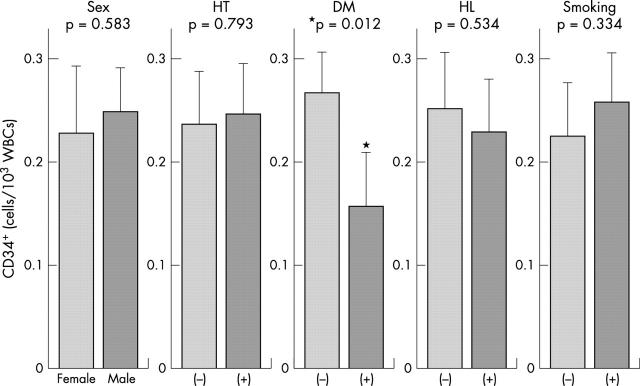
We then investigated the influence of risk factors on the number of circulating CD34+ cells. As shown in fig 2, univariate analysis identified diabetes mellitus as a significant predictor of a reduced CD34+ cell count. In contrast, the number of CD34+ cells did not significantly differ when patients were stratified according to sex, hypertension, hyperlipidaemia, and smoking.
Figure 2.
Effect of individual risk factors on the number of CD34+ cells. DM, diabetes mellitus; HL, hyperlipidaemia; HT, hypertension.
Multivariate analysis also demonstrated that diabetes mellitus is a significant independent predictor of a reduced circulating CD34+ cell count (standard coefficient −0.285, p = 0.027), whereas other factors were not significant.
DISCUSSION
The results of the present study demonstrate that the number of EPCs, as measured by the number of cells expressing CD34, was significantly reduced in patients with CAD compared with control subjects. Analysis of the individual risk factors indicated that subjects with diabetes mellitus had reduced numbers of circulating CD34+ cells. Given that several experimental studies indicate a significant contribution of EPCs for adult neovascularisation, the reduction in the number of CD34+ cells might contribute to reduced vascularisation in patients with CAD.
The mechanisms by which diabetes mellitus reduces CD34+ cell numbers remain to be determined. There are several possible scenarios by which diabetes mellitus could reduce the number of circulating CD34+ cells. One explanation might be increased apoptosis of premature progenitor cells. Indeed, CD34+ cells were shown to be very sensitive to apoptosis induction5 and diabetes mellitus is known to increase oxidative stress, a well established stimulus for apoptotic cell death. Alternatively, diabetes mellitus may interfere with the signalling pathways that regulate EPC differentiation or mobilisation. The present results might be related to premature atherosclerosis and impaired collateralisation in diabetes mellitus, although further studies are required to prove this hypothesis.
In conclusion, the present study demonstrates that CD34+ cell numbers are impaired in patients with CAD and this impairment is related to diabetes mellitus.
Abbreviations
CAD, coronary artery disease
EPC, endothelial progenitor cell
WBC, white blood cell
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–7. [DOI] [PubMed] [Google Scholar]
- 2.Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet 2002;360:427–35. [DOI] [PubMed] [Google Scholar]
- 3.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001;103:2776–9. [DOI] [PubMed] [Google Scholar]
- 4.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 2001;89:e1–e7. [DOI] [PubMed] [Google Scholar]
- 5.Ito H, Rovira II, Bloom ML, et al. Endothelial progenitor cells as putative targets for angiostatin. Cancer Res 1999;59:5875–7. [PubMed] [Google Scholar]