A 35 year old man was diagnosed as having Fabry disease because of extremely low activity of α-galactosidase A and a point mutation of its gene. He had been suffering from acroparesthesias, angiokeratoma, corneal opacity, and hypohidrosis. Since his neuropathic pain was resistant to conventional treatment, he agreed with written informed consent to participate in an open label trial of intravenous recombinant α-galactosidase A. He did not show overt heart or renal failure. However, he had cardiac hypertrophy and dysfunction. During six months of the trial, 0.2 mg/kg of α-galactosidase A was administered intravenously every other week.
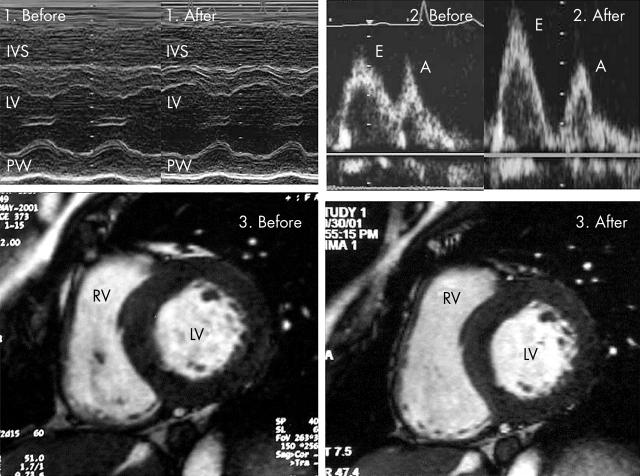
Echocardiography showed improvements in cardiac hypertrophy and systolic function: wall thickness decreased from 13 mm to 12 mm, fractional shortening increased from 31% to 35%, and left ventricular ejection fraction increased from 57% to 65% (panel 1). Furthermore, transmitral Doppler flow assessment confirmed improvements in diastolic function (panel 2). The peak flow velocity of the early filling (E) increased from 69 cm/s to 97 cm/s, and the E/A ratio increased from 1.1 to 1.6. Deceleration time of E decreased from 280 ms to 190 ms. Magnetic resonance imaging also revealed improvements in cardiac hypertrophy and left ventricular function: wall thickness decreased from 15 mm to 12 mm, left ventricular ejection fraction increased from 49% to 60%, and left ventricular mass index decreased from 110.8 g/m2 to 96.6 g/m2 and normalised (panel 3). These findings show that repeated intravenous administration of α-galactosidase A improved not only cardiac hypertrophy but also left ventricular systolic and diastolic function in Fabry disease.