Abstract
Objective: To determine how C reactive protein (CRP), a sensitive marker of inflammation, is related to coronary endothelial function.
Design: Changes in quantitative coronary angiographic findings and Doppler flow velocity measurements in response to locally infused acetylcholine were assessed.
Setting: Tertiary cardiology centre.
Patients: 46 patients with angiographically normal coronary arteries were divided into groups with normal (⩽ 3 mg/l) or increased (> 3 mg/l) CRP concentrations.
Interventions: Acetylcholine (3 and 30 μg/min) was infused into the left coronary ostium for two minutes.
Main outcome measures: Percentage change in diameter of epicardial coronary arteries and coronary blood flow (CBF) in response to acetylcholine; and correlations between these parameters and serum CRP concentrations.
Results: 15 patients had increased CRP concentrations. The change in coronary artery diameter induced by acetylcholine infusion was similar between the groups but the increase in CBF induced by acetylcholine was smaller in patients with increased CRP concentrations (54.9% v 139.4% with acetylcholine 30 μg/min, p = 0.0030). Multivariate analysis identified increased CRP concentration as independently associated with attenuated CBF response to acetylcholine at 30 μg/min (p = 0.0078, R2 = 0.434).
Conclusions: These findings suggest that inflammation appears to be associated with impaired coronary endothelial function in resistance but not conduit vessels. The data suggest a close relation between chronic vascular inflammation and endothelial dysfunction in atherosclerosis.
Keywords: coronary artery, inflammation, coronary vasoreactivity, acetylcholine, atherosclerosis
Much interest has focused on the relation between vascular inflammation and atherosclerosis.1–3 The hypothesised initial event is impairment of endothelial function by any of several factors. A subsequent inflammatory response leads to atherosclerotic plaque development and eventually atherosclerosis.1–3 Thus, endothelial function may be closely related to chronic low grade inflammation. Several clinical studies have investigated the relation between peripheral endothelial function and inflammation4–7 but much less is known about this relation in the coronary arteries.
C reactive protein (CRP), the prototypic acute phase reactant, is synthesised in the liver. A sensitive marker of the inflammatory response, CRP is measured readily and is widely used clinically.8–10 To determine whether an inflammatory response is associated with impaired coronary endothelial function, we correlated CRP concentration with coronary endothelial function as determined by the response to intracoronary infusions of acetylcholine.
METHODS
Study population
We studied 46 Japanese patients (mean age 59 years, 30 men and 16 women) who underwent coronary angiography for evaluation of atypical chest pain. All patients had angiographically normal epicardial coronary arteries, normal left ventricular function (contrast ventriculographic ejection fraction ⩾ 60%), and a normal coronary flow reserve (> 2.0). Patients with angiographically documented coronary spasm (> 50% luminal narrowing) after intracoronary injection of acetylcholine, left ventricular hypertrophy, or valvar heart disease were excluded from the study. Patients with obvious systemic inflammatory diseases or possible acute systemic inflammation as suggested by a CRP concentration > 10 mg/l also were excluded.11 No patient with unstable angina was included. Written informed consent was obtained from all patients before entry into the study. The protocol was approved by the ethics committee of our institution.
Study protocol
All antianginal agents were discontinued at least 48 hours before catheterisation, except for unrestricted use of sublingual glyceryl trinitrate, which was withheld for one hour before catheterisation. Diagnostic left heart catheterisation and coronary angiography were performed by a standard percutaneous brachial approach. A 6 French guide catheter was introduced into the left main coronary artery. A 0.0014 inch Doppler flow guidewire (FloWire, Cardiometrics, Mountain View, California, USA) was advanced through the guide catheter into the proximal segment of the left anterior descending coronary artery. The wire tip was positioned in a straight segment of the vessel to obtain a reliable flow velocity signal.
After baseline control conditions were established, incremental doses of acetylcholine were infused into the left coronary artery (3 and 30 μg/min) for two minutes, with five minute intervals between consecutive doses. After control conditions had been re-established, ATP (20 μg) was infused. Finally, an intracoronary infusion of glyceryl trinitrate was given at a rate of 200 μg/min for one minute. Acetylcholine and glyceryl trinitrate were infused directly into the left coronary ostium with an infusion pump (TE-311, Terumo, Tokyo, Japan) at a rate of 1 ml/min.
Coronary angiography was performed under control conditions and at the end of each drug infusion. Coronary blood flow (CBF) velocity was monitored continuously with a 12 MHz pulsed Doppler velocimeter (FloMap, Cardiometrics). Arterial pressure, heart rate, and ECG were monitored continuously and recorded with a multichannel recorder (Polygraph 1600, Nihon Electric Corporation, Tokyo, Japan).
Quantitative coronary angiography
The method for measuring coronary diameter has been described previously in detail.12,13 The coronary segment 2 mm distal to the Doppler wire tip was selected for quantitative analysis. In each patient, luminal diameters of selected segments of the left anterior descending coronary artery were measured by a single investigator blinded to angiographic and clinical data to determine the effects of different drugs on the epicardial coronary diameter. Luminal diameters were measured on an end diastolic frame by a computer assisted coronary angiographic analysis system (CAAS II/QUANTCOR, Siemens, Berlin, Germany). Means of triplicate measurements of luminal diameter were used for analysis. Changes in coronary diameter in response to acetylcholine and glyceryl trinitrate infusion are expressed as percentage change from the baseline measurement on the angiogram obtained before infusion. Intraobserver and interobserver variability previously were shown to be excellent.12,13
Estimation of CBF and coronary flow reserve
CBF was calculated as the product of CBF velocity and vessel diameter according to the following formula: π × average peak velocity × 0.125 × diameter2. For CBF calculations, the internal diameter of the vessel at the location of the flow measurements (2 mm distal to the wire tip) was measured by the method described above. Coronary flow reserve was calculated as the ratio of CBF velocity after an injection of ATP to the baseline velocity.
Biochemical parameters
Fasting blood samples were obtained just before coronary angiography was performed. Routine methods were used to determine serum concentrations of standard biochemical parameters. CRP concentration was determined by a high sensitivity CRP test (N Latex CRP II, Dade Behring, Marburg, Germany). According to the manufacturer’s information, the limit of detection in this assay is 0.02 mg/l. The reported intra-assay coefficient of variation for high serum CRP concentrations is 1.5–2.4%; for intermediate serum CRP concentrations, 2.8–4.7%; and for low serum CRP concentrations, 1.5–4.6%. The mean values of the coefficient of variation for measurements obtained over four days for high, moderate, and low serum CRP concentrations were 2.0%, 2.4%, and 2.8%, respectively. Patients were divided into two groups depending on whether the CRP concentration was normal (⩽ 3 mg/l) or increased (> 3 mg/l).
Statistical analysis
All data are expressed as the mean (SEM). Baseline characteristics between the two groups were compared by Student’s unpaired t test or the χ2 analysis, as appropriate. Serial changes in haemodynamic variables and changes in coronary vasoreactivity in response to drug infusion were compared by a one way analysis of variance. If the analysis of variance showed a significant difference between means, the level of significance was determined by contrast analysis. Serial percentage changes in the coronary vascular response to acetylcholine infusion were compared between groups by a two way analysis of variance. We performed univariate and multivariate regression analyses to identify factors associated with percentage changes in CBF induced by acetylcholine. A probability value of p < 0.05 was considered to indicate significance.
RESULTS
Patient characteristics and biochemical parameters
Table 1 summarises the characteristics of patients. Fifteen patients had increased CRP concentrations. Distributions of age, sex, and body mass index in the two groups were similar. Frequencies of individual coronary risk factors and the numbers of patients who were taking aspirin or statins were also similar. Biochemical analysis showed that total cholesterol and low density lipoprotein cholesterol concentrations were higher, and high density lipoprotein cholesterol concentrations tended to be higher, in patients with increased CRP concentrations. The CRP concentration was 5.05 (0.33) mg/l in patients with increased CRP concentrations and 0.35 (0.23) mg/l in patients with normal CRP concentrations (p < 0.0001).
Table 1.
Clinical characteristics of study patients
| Increased CRP (n = 15) | Normal CRP (n = 31) | p Value | |
| Age (years) | 61 (3) | 59 (2) | NS |
| Men/women | 8/7 | 22/9 | NS |
| Body mass index (kg/m2) | 24.8 (0.8) | 24.6 (0.6) | NS |
| Coronary risk factors | |||
| Smoking | 5 (33%) | 8 (26%) | NS |
| Hypertension* | 5 (33%) | 15 (48%) | NS |
| Hypercholesterolaemia† | 8 (53%) | 10 (32%) | NS |
| Total cholesterol (mmol/l) | 5.86 (0.22) | 5.11 (0.15) | 0.0079 |
| Triglycerides (mmol/l) | 1.85 (0.19) | 1.52 (0.13) | NS |
| HDL cholesterol (mmol/l) | 1.27 (0.10) | 1.49 (0.07) | 0.0848 |
| LDL cholesterol (mmol/l) | 3.74 (0.21) | 2.92 (0.14) | 0.0024 |
| Diabetes mellitus | 2 (13%) | 4 (13%) | NS |
| Serum glucose (mmol/l) | 5.43 (0.28) | 5.65 (0.20) | NS |
| Medications | |||
| Aspirin | 7 (43%) | 13 (42%) | NS |
| Statin | 3 (20%) | 5 (16%) | NS |
| High sensitive CRP (mg/l) | 5.05 (0.33) | 0.34 (0.23) | <0.0001 |
Data are mean (SEM) unless otherwise indicated.
*Taking antihypertensive treatment or having blood pressure 140/90 mm Hg; †taking cholesterol lowering treatment or having total cholesterol 6.21 mmol/l.
CRP, C reactive protein; HDL, high density lipoprotein; LDL, low density lipoprotein; NS, not significant.
CRP concentration and coronary vasoreactivity
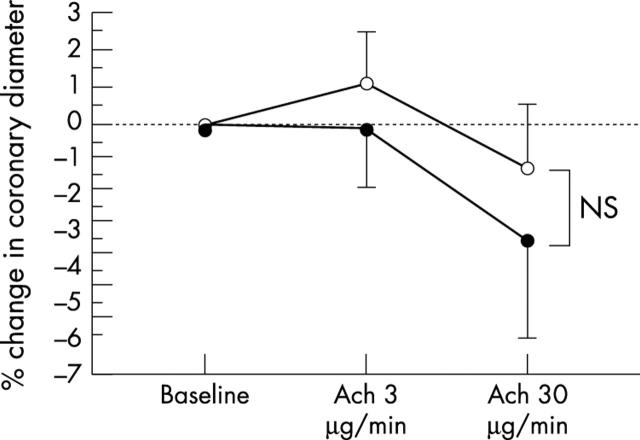
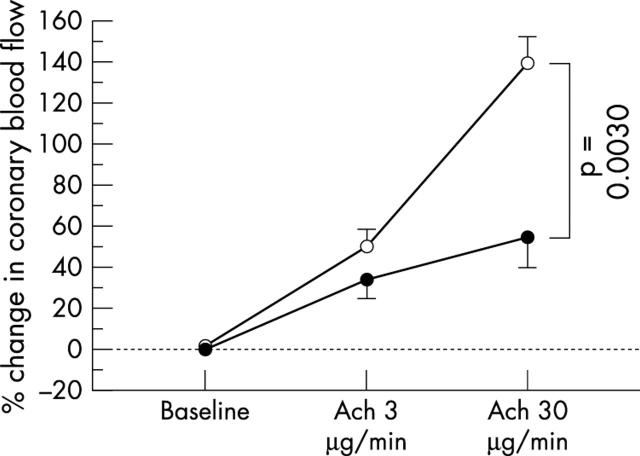
Baseline heart rate and mean arterial pressure in the two groups were similar before and during acetylcholine infusion. Glyceryl trinitrate infusion increased heart rate and decreased mean arterial pressure. Coronary artery diameter and CBF at baseline were similar between the two groups. Changes in the coronary diameter in response to acetylcholine infusion also were similar between the groups (fig 1 and table 2), but the increase in CBF in response to acetylcholine infusion was attenuated in patients with increased CRP concentrations relative to that in patients with normal CRP concentrations (p = 0.0030; fig 2 and table 2). Coronary flow reserve was similar between the two groups (table 2), as was glyceryl trinitrate induced change in coronary diameter (table 2). Because the difference in coronary vasoreactivity between the two groups was greatest after infusion of acetylcholine at a dose of 30 μg/min, in subsequent analyses we used the increase obtained with that dose.
Figure 1.
Percentage changes in epicardial coronary artery diameter in response to acetylcholine (ACh) infusion. The changes in coronary artery diameter in response to Ach infusion were similar between patients with increased C reactive protein (CRP) concentrations (solid circles) and patients with normal CRP concentrations (open circles). Vertical bars represent SEM. NS, not significant.
Table 2.
Haemodynamic variables and coronary vasoreactivity
| Increased CRP | Normal CRP | |||
| Value | % change | Value | % change | |
| Baseline mean blood pressure (mm Hg) | 112 (4) | 107 (3) | ||
| Baseline heart rate (beats/min) | 68 (3) | 67 (2) | ||
| Coronary diameter (mm) | ||||
| Baseline | 3.12 (0.14) | 0 | 3.12 (0.10) | 0 |
| Acetylcholine 3 μg/min | 3.13 (0.15) | −0.1 (1.9) | 3.15 (0.10) | 1.1 (1.3) |
| Acetylcholine 30 μg/min | 3.03 (0.16) | −3.3 (2.5) | 3.08 (0.11) | −1.2 (1.8) |
| Glyceryl trinitrate | 3.54 (0.13)* | 15.3 (3.4)* | 3.51 (0.09)* | 13.0 (2.4)* |
| Coronary blood flow (ml/min) | ||||
| Baseline | 68.5 (8.6) | 0 | 79.0 (6.0) | 0 |
| Acetylcholine 3 μg/min | 93.5 (15.3)† | 34.0 (11.7)† | 118.1 (10.6)† | 50.6 (8.2)† |
| Acetylcholine 30 μg/min | 107.8 (21.7)*‡ | 54.9 (18.8)*‡ | 187.7 (15.1)* | 139.4 (13.1)* |
| Coronary flow reserve | 3.12 (0.21) | 3.28 (0.15) | ||
Data are mean (SEM).
*p<0.01 v others; †p<0.01 v baseline; ‡p<0.01 v normal CRP.
Figure 2.
Percentage change in coronary blood flow (CBF) in response to ACh infusion. The increase in CBF in response to Ach infusion was attenuated in patients with increased CRP concentrations (solid circles) relative to that in patients with normal CRP concentrations (open circles). Vertical bars represent SEM.
Factors associated with impaired coronary vasoreactivity
On the basis of univariate analysis, factors associated with the increase in CBF induced by acetylcholine at 30 μg/min were male sex (p = 0.0157), increased CRP concentration (p = 0.0006), and coronary flow reserve (p = 0.0119). In addition, factors associated at p < 0.2 with the increase in CBF induced by acetylcholine at 30 μg/min were serum concentrations of triglyceride (p = 0.1156), high density lipoprotein cholesterol (p = 0.1120), and low density lipoprotein cholesterol (p = 0.1932). Multivariate regression analysis of these factors showed that increased CRP concentration (t = −2.81, p = 0.0078) and coronary flow reserve (t = 2.28, p = 0.0279) were associated with the increase in CBF induced by acetylcholine at 30 μg/min (R2 = 0.434).
DISCUSSION
The present study investigated the relation between coronary endothelial function and serum concentrations of CRP, a marker of inflammation, in patients with atypical chest pain and normal coronary arteries. We found that the acetylcholine induced increase in CBF was reduced in patients with increased CRP concentrations. Furthermore, multivariate regression analysis indicated that increased CRP concentration was independently associated with attenuation of the increase in CBF in response to acetylcholine infusion. Our results suggest that chronic low grade inflammation may be associated with microvascular coronary endothelial dysfunction.
Previous studies on endothelial function and the inflammatory response
Atherosclerosis has been suggested to be an inflammatory disease1–3,14 initiated by endothelial dysfunction caused by several risk factors. Subsequent vascular inflammation leads to an increased CRP concentration, either secondarily or with CRP as a direct participant in the inflammatory process,15 attenuating nitric oxide production in the endothelium.16 Irrespective of pathogenetic issues concerning CRP, several studies have established a close relation between endothelial function and the inflammatory response.4–7 Fichtlscherer et al4 and Sinisalo et al6 have shown that CRP concentrations are associated with the intensity of the response of forearm blood flow to acetylcholine infusion in patients with coronary artery disease. Cleland et al5 have shown that the CRP concentration correlates with basal forearm blood flow in healthy patients by using NG-monomethyl-l-arginine, a nitric oxide synthase inhibitor. These findings suggest that the inflammation is associated with impaired endothelial function in resistance vessels, although differences in study populations may have contributed to basal or agonist induced endothelial dysfunction. In addition, Paisley et al7 observed endothelial dysfunction in conduit vessels in patients with asymptomatic proteinuria, although an inflammatory response was found only in resistance vessels. Thus, a low grade inflammatory response seems to be associated with impaired endothelial function, at least in resistance vessels. However, several studies examined relations between the inflammatory response and peripheral endothelial function. Only a single investigation addressed the relation between the inflammatory response and coronary vasoreactivity; that study was of patients with unstable angina.17
Inflammatory response and coronary endothelial function
Tomai et al17 reported that increased CRP concentrations were related to attenuation of changes in minimal epicardial coronary artery diameter during the cold pressor test in patients with unstable angina; this suggested that increased CRP concentration was associated with enhanced vasoreactivity at the site of the culprit lesion. Several modulators may affect vasoreactivity at the involved site in unstable angina and endothelial function presumably was not the sole influence on local coronary vasoreactivity. On the other hand, when we investigated the relation between CRP concentration and acetylcholine induced coronary vasoreactivity in patients with atypical chest pain and normal coronary arteries, we were able to investigate the relation between the inflammatory response and coronary endothelial function without the complexities introduced by an established lesion.
The present study showed that CRP concentrations were not associated with acetylcholine induced change in coronary diameter but did influence the degree of acetylcholine induced increase in CBF independently of coronary flow reserve. In previous studies,18,19 the acetylcholine induced increase in CBF was reduced by an infusion of NG-monomethyl-l-arginine, indicating that endothelial nitric oxide has at least some role in the CBF response to acetylcholine infusion. Thus, our results suggest that an inflammatory response is associated with coronary endothelial dysfunction at the level of resistance vessels. The exact mechanisms linking CRP concentration with microvascular endothelial function are unclear. The mean CRP concentration in this study was less increased than that observed in the report17 concerning unstable angina; indeed, because CRP is increased in patients with unstable angina,20 we excluded patients with this possible diagnosis. In addition, our study population had no evidence of atherosclerotic lesions on angiography. Such differences may have influenced our results.
Relation between the CRP concentration and other parameters
In the present study, total cholesterol and low density lipoprotein cholesterol concentrations were increased in patients with increased CRP concentrations, essentially in agreement with previous reports.21,22 The reasons why CRP concentration correlates with lipid concentrations are unclear. However, lipid increase is a coronary risk factor and is known to impair endothelial function.13,23,24 Since CRP is a marker of endothelial dysfunction, CRP may correlate with lipids on that basis. On the other hand, our results did not show a relation between CRP and body mass index, as reported from other studies.21,25 Our subject population was not obese, which may account for this difference.
Recently, much interest has focused on the anti-inflammatory effect of statins.26,27 In addition, use of low dose aspirin may reduce CRP concentrations28,29 in patients with coronary artery disease, while aspirin has no effect on CRP in healthy patients.30 The present study failed to find any relation between the use of these drugs and CRP concentration or coronary vasoreactivity. Differences in patient characteristics and in doses and duration of drug treatment may account for a lack of a drug effect here.
Study limitations
Several limitations to the present study should be considered. Firstly, the relatively small number of patients may have obscured relations between CRP concentration and some other variables (type II error). Secondly, while our study population was a homogeneous group with atypical chest pain, angiographically normal coronary arteries, normal left ventricular function, and no vasospastic or unstable angina, a wholly homogeneous underlying disease state cannot be guaranteed. Occult variability, then, may have contributed to variability in CRP concentration. Thirdly, in the measurement of CRP, the use of two samples has been recommended,11 but we measured CRP only once. In addition, we divided patients into two groups depending on whether the CRP concentration was normal (⩽ 3 mg/l) or increased (> 3 mg/l). However, patients with a CRP concentration of 1.0–3.0 mg/l, who would fall into our “normal CRP concentration” group, have been shown to have a moderate relative risk of future cardiovascular events.11 Therefore, we need to reassess the relation between coronary endothelial function and CRP concentration based on the relative risk categories.11 Lastly, patients with obvious systemic inflammation or acute infection were excluded from the study, since systemic inflammation or acute infection may affect not only CRP concentration but also endothelial function.31–33 Yet we did not perform a thorough medical investigation aimed at uncovering all possible sources of inflammation, so increased CRP concentrations may have resulted from systemic inflammation in some patients.
Conclusions
Our present findings indicate that a chronic low grade inflammatory response is associated with microvascular coronary endothelial dysfunction. These findings support a close relation between endothelial dysfunction and vascular inflammation in the pathogenesis of atherosclerosis.
Acknowledgments
We are grateful to Fumiharu Miura MD, Sou Takenaka MD, and Masayuki Katsumoto MD of the Department of Medicine and Molecular Science, Graduate School of Biomedical Sciences, Hiroshima University, for their technical assistance and helpful comments. We also thank Yuko Omura for her secretarial assistance.
REFERENCES
- 1.Ross R . Atherosclerosis: an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 2.Kinlay S , Libby P, Ganz P. Endothelial function and coronary artery disease. Curr Opin Lipidol 2001;12:383–9. [DOI] [PubMed] [Google Scholar]
- 3.Libby P . Inflammation in atherosclerosis. Nature 2002;420:868–74. [DOI] [PubMed] [Google Scholar]
- 4.Fichtlscherer S , Rosenberger G, Walter DH, et al. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation 2000;102:1000–6. [DOI] [PubMed] [Google Scholar]
- 5.Cleland SJ, Sattar N, Petrie JR, et al. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin Sci (Colch) 2000;98:531–5. [PubMed] [Google Scholar]
- 6.Sinisalo J , Paronen J, Mattila KJ, et al. Relation of inflammation to vascular function in patients with coronary heart disease. Atherosclerosis 2000;149:403–11. [DOI] [PubMed] [Google Scholar]
- 7.Paisley KE, Beaman M, Tooke JE, et al. Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int 2003;63:624–33. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001;103:1813–8. [DOI] [PubMed] [Google Scholar]
- 11.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 12.Teragawa H , Kato M, Yamagata T, et al. Magnesium causes nitric oxide independent coronary artery vasodilation in humans. Heart 2001;86:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda Y , Teragawa H, Matsuda K, et al. Tetrahydrobiopterin restores endothelial function of coronary arteries in patients with hypercholesterolaemia. Heart 2002;87:264–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libby P . Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001;104:365–72. [DOI] [PubMed] [Google Scholar]
- 15.Bharadwaj D , Stein MP, Volzer M, et al. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med 1999;190:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S , Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002;106:913–9. [DOI] [PubMed] [Google Scholar]
- 17.Tomai F , Crea F, Gaspardone A, et al. Unstable angina and elevated c-reactive protein levels predict enhanced vasoreactivity of the culprit lesion. Circulation 2001;104:1471–6. [DOI] [PubMed] [Google Scholar]
- 18.Quyyumi AA, Dakak N, Mulcahy D, et al. Nitric oxide activity in the atherosclerotic human coronary circulation. J Am Coll Cardiol 1997;29:308–17. [DOI] [PubMed] [Google Scholar]
- 19.Tagawa T , Mohri M, Tagawa H, et al. Role of nitric oxide in substance P-induced vasodilation differs between the coronary and forearm circulation in humans. J Cardiovasc Pharmacol 1997;29:546–53. [DOI] [PubMed] [Google Scholar]
- 20.Versaci F , Gaspardone A, Tomai F, et al. Predictive value of C-reactive protein in patients with unstable angina pectoris undergoing coronary artery stent implantation. Am J Cardiol 2000;85:92–5, A8. [DOI] [PubMed] [Google Scholar]
- 21.Chambers JC, Eda S, Bassett P, et al. C-reactive protein, insulin resistance, central obesity, and coronary heart disease risk in Indian Asians from the United Kingdom compared with European whites. Circulation 2001;104:145–50. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363–9. [DOI] [PubMed] [Google Scholar]
- 23.Shiode N , Nakayama K, Morishima N, et al. Nitric oxide production by coronary conductance and resistance vessels in hypercholesterolemia patients. Am Heart J 1996;131:1051–7. [DOI] [PubMed] [Google Scholar]
- 24.Capell WH, DeSouza CA, Poirier P, et al. Short-term triglyceride lowering with fenofibrate improves vasodilator function in subjects with hypertriglyceridemia. Arterioscler Thromb Vasc Biol 2003;23:307–13. [DOI] [PubMed] [Google Scholar]
- 25.Lemieux I , Pascot A, Prud’homme D, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 2001;21:961–7. [DOI] [PubMed] [Google Scholar]
- 26.Horne BD, Muhlestein JB, Carlquist JF, et al. Statin therapy, lipid levels, C-reactive protein and the survival of patients with angiographically severe coronary artery disease. J Am Coll Cardiol 2000;36:1774–80. [DOI] [PubMed] [Google Scholar]
- 27.Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA 2001;286:64–70. [DOI] [PubMed] [Google Scholar]
- 28.Ikonomidis I , Andreotti F, Economou E, et al. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation 1999;100:793–8. [DOI] [PubMed] [Google Scholar]
- 29.Kennon S , Price CP, Mills PG, et al. The effect of aspirin on C-reactive protein as a marker of risk in unstable angina. J Am Coll Cardiol 2001;37:1266–70. [DOI] [PubMed] [Google Scholar]
- 30.Feldman M , Jialal I, Devaraj S, et al. Effects of low-dose aspirin on serum C-reactive protein and thromboxane B2 concentrations: a placebo-controlled study using a highly sensitive C-reactive protein assay. J Am Coll Cardiol 2001;37:2036–41. [DOI] [PubMed] [Google Scholar]
- 31.Raza K , Thambyrajah J, Townend JN, et al. Suppression of inflammation in primary systemic vasculitis restores vascular endothelial function: lessons for atherosclerotic disease? Circulation 2000;102:1470–2. [DOI] [PubMed] [Google Scholar]
- 32.Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation 2000;102:994–9. [DOI] [PubMed] [Google Scholar]
- 33.Kharbanda RK, Walton B, Allen M, et al. Prevention of inflammation-induced endothelial dysfunction: a novel vasculo-protective action of aspirin. Circulation 2002;105:2600–4. [DOI] [PubMed] [Google Scholar]