Abstract
Background: Atrial en-face reconstructions are commonly used to assess mitral valve morphology in three dimensional (3D) echocardiography but may miss important abnormalities.
Objective: To present a systematic method for the analysis of the regurgitant mitral valve using a combination of en-face and longitudinal views for better anatomical evaluation.
Methods: Detailed 3D assessment was done on 58 patients undergoing mitral valve repair. En-face and longitudinal views were compared for detection and location of primary pathology. The quality of acquisitions under general anaesthesia and sedation was also compared.
Results: Recognition of valve structure was significantly better with longitudinal reconstruction for both mitral leaflets but not for the commissures. Accurate identification of pathology was possible in 95% cases, compared with 50% for en-face reconstruction (p < 0.001). There was no significant difference between imaging under sedation and anaesthesia.
Conclusion: En-face reconstructions alone are inadequate. Additional longitudinal reconstructions are necessary to ensure full inspection of valve morphology.
Severe mitral regurgitation carries a high cost for patients in terms of mortality1–4 and morbidity.2,5–7 Mitral valve repair has been substituted for valve replacement as the procedure of choice for isolated regurgitation, and the timing of intervention is critically linked to valve reparability. The feasibility of repair depends on the anatomical lesion,8–12 and knowledge of the involved leaflet segments is very important for the surgeon.13,14
Three dimensional (3D) echocardiography has been applied to the evaluation of mitral valve morphology15–21 and may play a valuable role in the assessment of patients undergoing mitral repair surgery.17,18,20,21 The standard view for assessing the valve with this technique is the left atriotomy or surgeons’ eye view.17–23 However, in our experience this view is not always optimal. This could affect decisions about suitability for valve repair and therefore about the timing of surgery. Arbitrary longitudinal views have been used in some studies17,19,24,25 to enable a better assessment of the valve, but to our knowledge en-face and longitudinal viewing planes have not been systematically evaluated. We therefore compared the atrial en-face view with serial longitudinal reconstructions to determine whether the latter might add additional information on valve structure and function.
METHODS
Study population
Adult patients were recruited to undergo 3D acquisition if they fulfilled the following criteria:
they had severe mitral regurgitation based on semiquantitative assessment26–29
they were suitable for mitral valve repair
they had had routine diagnostic transoesophageal echocardiography (TOE) at our institution
they tolerated 3D acquisition.
Patients were excluded if they had associated mitral stenosis or if their 3D acquisition was unsuccessful. Following appropriate consent, patients underwent standard multiplane TOE either under sedation during a routine TOE list in the outpatient setting, or under general anaesthesia in the operating room immediately before surgery and on one occasion in the intensive care unit. Whenever possible ventilated patients had the 3D acquisition during apnoea following preoxygenation with 100% oxygen and continuous pulse oximetry under the direct supervision of a senior cardiac anaesthetist. The patient’s clinical condition allowed apnoea in all but three cases, including the patient in intensive care mentioned above. These three patients had acquisitions while they were having intermittent positive pressure ventilation.
Aetiology of mitral regurgitation
The gross aetiology of the mitral disease was classified as degenerative, endocarditic, or functional. Applying the Carpentier nomenclature,30 the function of all eight segments of the valve (that is, the lateral scallop of anterior leaflet (A1), the middle scallop of anterior leaflet (A2), the medial scallop of anterior leaflet (A3), the lateral scallop of posterior leaflet (P1), the middle scallop of posterior leaflet (P2), the medial scallop of posterior leaflet (P3), the antero-lateral commissure (ALC), and the postero-medial commissure (PMC)) was classified by the operating surgeon as normal, prolapsing, flail, tethered, perforated, or eroded, and the primary mechanism of mitral regurgitation was recorded.
Acquisition method
A commercially available echo system was used for image acquisition (Hewlett Packard 5500, 5 MHz multiplane TOE probe and 3D acquisition software; Hewlett Packard Inc, Andover, Massachusetts, USA). The 3D examination was done after a routine diagnostic TOE with 3° rotational scanning of the mitral valve undertaken from a mid or low oesophageal window. The ECG gates were set with RR intervals below 200 ms in the expiratory phase of the respiratory cycle. The images were digitised and stored on magneto-optical discs for off line analysis. Only one complete acquisition was attempted for each patient.
Reconstruction method
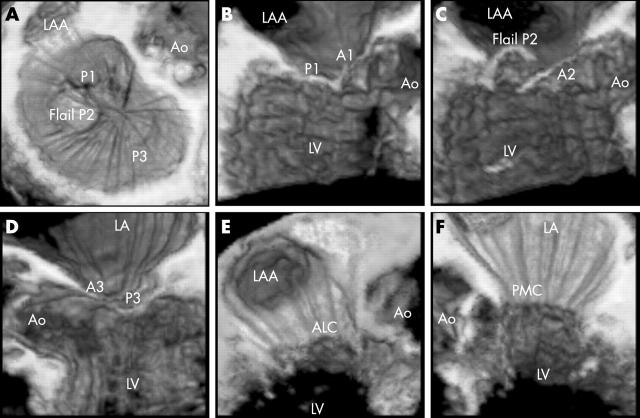
Using a voxel based 3D work station (Echoscan 4.1, TomTec Imaging Systems GmbH, Munich, Germany), a volume rendered en-face view of the mitral valve from the left atrium (the surgeon’s eye view) was reconstructed for each patient. Guided by this view, five serial longitudinal cut planes were then reconstructed to visualise A1–P1, A2–P2, A3–P3, and the two commissures (fig 1, panels A to D). An example of a complete volume rendered 3D mitral study with all six views is presented in fig 2.
Figure 1.
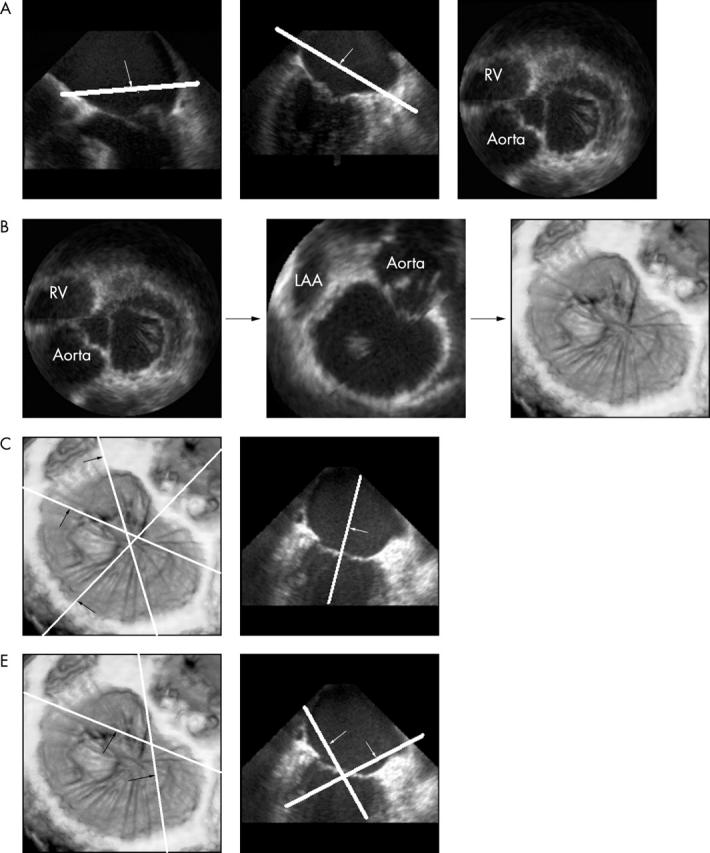
(A) Cut planes are made in the orthogonal four and two chamber two dimensional (2D) views parallel to the plane of the mitral annulus, allowing 2D visualisation of the valve from above. (B) This image is rotated to represent the surgeon’s eye view and magnified to optimise the depiction of the mitral valve. This is volume rendered to a three dimensional (3D) image. (C) Three cut planes are made through A1/P1, A2/P2, and A3/P3 as shown. Keeping the perpendicular plane along the long axis of the ventricle, corresponding longitudinal images are then surface rendered to show the valve leaflets optimally. (D) The commissural zones are visualised similarly but with an oblique plane looking down onto the valve from the atrium. A1/P1, lateral scallops of anterior and posterior mitral leaflets; A2/P2, middle scallops of anterior and posterior leaflets; A3/P3, medial scallops of anterior and posterior leaflets; LAA, left atrial appendage; RV, right ventricle.
Figure 2.
The six views used for systematic three dimensional (3D) assessment of the mitral valve. (A) En-face view of the mitral valve. (B) Longitudinal view with a cut made through A1/P1. (C) Cut through A2/P2. (D) Cut through A3/P3. (E) Oblique view showing the antero-lateral commissure. (F) Oblique view showing the postero-medial commissure. In this example, a flail P2 segment can be seen easily in the en-face view, but this particular valve reconstruction was chosen for its clarity for the purpose of demonstration. A1/P1, lateral scallops of anterior and posterior mitral valve leaflets; A2/P2, middle scallops of anterior and posterior leaflets; A3/P3, medial scallops of anterior and posterior leaflets; ALC, antero-lateral commissure; Ao, aorta; LA, left atrium; LAA, left atrial appendage; LV, left ventricle; PMC, postero-medial commissure.
Scoring protocol
The atrial en-face and longitudinal views were analysed separately by a single experienced observer blinded to the surgical findings. A three point scoring system (0, inadequate for analysis; 1, adequate; 2, good) was applied to all eight segments of the mitral valve leaflets, according to ease of recognition and confidence of interpretation of function. The maximum score obtainable per view for each valve was 16.
In addition, the observer recorded the location and morphology of the primary pathological segment from each view. The accuracy of this assessment was scored as positive or negative, using the surgical findings as the gold standard. This ability to identify the primary mechanism of mitral regurgitation was compared for the two types of view. To calculate the interobserver variability in scores, an inexperienced operator also analysed both views from 20 randomly selected 3D studies.
Statistics
Baseline patient characteristics and acquisition times are presented as mean (SD). The recognition scores are expressed as median (interquartile range). The time taken for the 3D procedures in the sedation and anaesthesia groups was compared using the Mann–Whitney test for independent non-parametric data. The Wilcoxon signed rank test for paired non-parametric data was used for comparison of recognition scores between the two types of view. The χ2 test was used to compare the ability of the two forms of reconstruction to identify the aetiology of mitral regurgitation. Interobserver correlations were made using the Spearman ρ test. Two tailed statistical tests were used, and a probability value of p < 0.05 was considered significant. All statistical analysis was undertaken using SPSS 10.1 for windows.
RESULTS
Baseline characteristics
Over the study period 70 patients underwent diagnostic TOE before mitral valve repair. Twelve patients (17%) were excluded because of unsuccessful 3D acquisition. All failed acquisitions were attempts during TOE under sedation. The reasons for failure included movement artefacts caused by coughing or hiccups, difficulty with ECG gating, and equipment failure. For the remaining 58 patients included in the study, the mean (SD) age was 61 (12) years (range 22–79 years), 64% were male, and 74% were in sinus rhythm. Twenty nine had the procedure under sedation and 29 under general anaesthesia. Eleven of the 58 patients had the procedure twice—that is, under sedation and also under anaesthesia.
Aetiology of mitral regurgitation
The gross aetiological classification of mitral regurgitation was degenerative disease in 74%, functional in 16%, and endocarditic in 10%. The primary cause of mitral regurgitation was flail leaflet in 23 cases, prolapse in 21, tethering in nine, perforation of leaflet body in three, erosion of leaflet edge in three, and annular dilatation with normal leaflet function in four. The two middle scallops of the valve were most often involved in the primary mechanism of mitral regurgitation (P2 in 28 patients, A2 in 12). The majority of patients had less than three scallops that were morphologically abnormal (one scallop in 23 patients and two in 18).
Time taken to acquire and reconstruct in 3D
Of the 58 cases, the exact time for image acquisition was available in 54. The mean (SD) acquisition time was 3.83 (2.05) mins overall. Acquisitions during sinus rhythm (43 studies) were shorter than during atrial fibrillation, but not significantly so (3.43 (1.41) mins for sinus rhythm v 5.41 (3.22) mins for atrial fibrillation; p = 0.066). Acquisition times for 3D under anaesthesia were significantly shorter than under sedation (2.55 (0.56) mins under anaesthesia v 5.32 (2.14) mins with sedation; p < 0.001). The reconstruction time calculated for a random selection of 20 cases was 19.41 (7.6) mins (range 5.45–28.42 mins).
Recognition scores for mitral valve segments
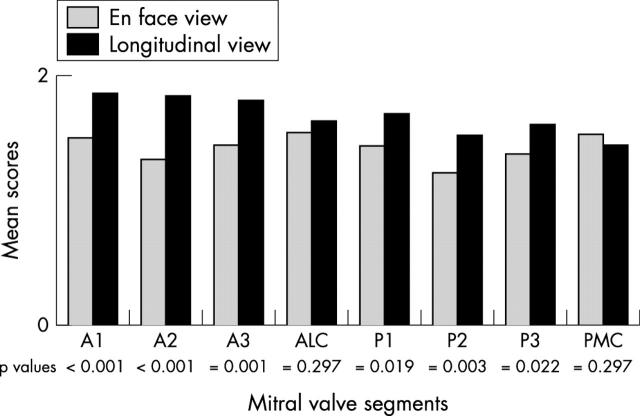
Total scores were significantly better for longitudinal reconstructions than for en-face atrial views (median score (interquartile range), 13.50 (12–15) v 12.00 (9–15); p < 0.001). Longitudinal reconstructions allowed more accurate identification of both individual leaflets (median score (interquartile range), 6.00 (5–6) v 4.50 (3–6), p < 0.001, for anterior leaflet; 5.00 (4–6) v 4.00 (3–5), p < 0.001, for posterior leaflet) but offered no advantage for the commissures. Figure 3 demonstrates this and shows detailed segmental analysis for all the eight valve segments.
Figure 3.
Comparison of segmental visualisation scores obtained by en-face and longitudinal three dimensional reconstruction. There was a significant incremental value in the longitudinal views for all leaflet segments but not the commissures (maximum score obtainable for each segment per view was 2). A1, lateral scallop of anterior leaflet; A2, middle scallop of anterior leaflet; A3, medial scallop of anterior leaflet; ALC, anterolateral commissure; P1, lateral scallop of posterior leaflet; P2, middle scallop of posterior leaflet; P3, medial scallop of posterior leaflet; PMC, postero-medial commissure.
Detection of primary cause of mitral regurgitation
Using surgical findings as the gold standard, 3D reconstructions were able to identify the primary pathology in 95% of cases when all six views were combined, but longitudinal views were superior to the en-face views (en-face views accurate in 50% of cases, compared with 95% cases by longitudinal views; p < 0.001). Of the 29 patients in whom the en-face view was inadequate, pathology could be identified accurately by the longitudinal views in 26. By contrast, in the three patients in whom longitudinal views were inaccurate, the en-face views did not add any further information.
Effect of functional classification and location of pathology on accuracy of detection
Table 1 presents the primary causes of mitral regurgitation diagnosed by the surgeon, and the ability of both methods of reconstruction to detect these pathologies. Longitudinal views were significantly better at identifying the majority of primary causes (p = 0.008, p = 0.002, and p = 0.025 for flail, prolapsed, and tethered segments, respectively). This superiority was seen regardless of the location of the primary lesion (p values for the most common locations were 0.002 for P2 and 0.008 for A2) or the number of involved segments (p = 0.001 for single segment pathology and p = 0.014 for two segments).
Table 1.
Detection of pathology
| Primary cause of mitral regurgitation | Total number of segments | En-face view | Longitudinal view | ||
| Accurate detection | Inaccurate detection | Accurate detection | Inaccurate detection | ||
| Flail | 23 | 15 (65%) | 8 (35%) | 22 (96%) | 1 (4%) |
| Prolapse | 20 | 10 (50%) | 10 (50%) | 19 (95%) | 1 (5%) |
| Tethering | 9 | 4 (44%) | 5 (56%) | 9 (100%) | 0 (0%) |
| Perforation | 3 | 1 (33%) | 2 (67%) | 3 (100%) | 0 (0%) |
| Erosion | 3 | 1 (33%) | 2 (67%) | 2 (67%) | 1 (33%) |
The table shows the superiority of longitudinal views over en-face views in the accurate detection of functional pathology. Values represent numbers of segments and percentage of total number of segments with that morphology.
Comparison of general anaesthesia with sedation for scores and pathology
There was no difference in the baseline characteristics of patients who had 3D assessment under sedation versus anaesthesia (that is, there was no statistical difference between sedation and general anaesthesia for age, sex, rhythm, aetiology of valve disease, primary mechanism of mitral regurgitation, or location and extent of pathology). Comparison of these two groups showed no significant differences in the total recognition scores (median score (interquartile range), 12.00 (6.75–14.50) for sedation v 12.00 (9–15) for general anaesthesia (p = 0.466) with en-face views; and 13.00 (12–16) for sedation v 14.00 (13–14) for general anaesthesia (p = 0.450) with longitudinal views). There was, however, a trend towards more accurate detection of the mechanism of regurgitation under sedation (59% accurate detection by sedation v 41% by general anaesthesia (p = 0.193) for en-face views; 100% by sedation v 89% by general anaesthesia (p = 0.078) for longitudinal views).
In the 11 patients who had both types of 3D acquisition there was no difference between sedation and anaesthesia (median score (interquartile range), 12.00 (9–13) for sedation v 9.00 (8–14) for general anaesthesia (p = 0.699) for en-face views; and 13.00 (12–16) for sedation v 14.00 (12–15) for general anaesthesia (p = 0.606) for longitudinal views).
Interobserver variability
Twenty 3D studies were randomly selected and evaluated by an operator with no experience in 3D imaging. There was a trend towards closer agreement in recognition scores between observers with the longitudinal views (r = 0.425, p = 0.062) than with the en-face views (r = 0.374, p = 0.104). Agreement on pathology detection, however, was significantly better for longitudinal views (r = 0.840, p < 0.001) than for en-face views (r = 0.414, p = 0.069).
DISCUSSION
In this study we developed a systematic approach to the 3D evaluation of the regurgitant mitral valve based on the anatomy and surgical classification of the mechanisms of mitral regurgitation. We studied an unselected cohort of patients undergoing mitral valve repair with a representative spread of aetiologies that are typically encountered at our surgical centre. We formulated a series of longitudinal views by slicing the en-face view perpendicularly, and compared the two types of reconstruction for ease of recognition of the segments of the mitral valve according to the Carpentier classification, and also for the detection of pathology. The positioning of the longitudinal cut planes can be modified to accommodate the Duran classification14,31 as well. This method would be equally applicable to transthoracic 3D assessment of the mitral valve, assuming adequate image quality.
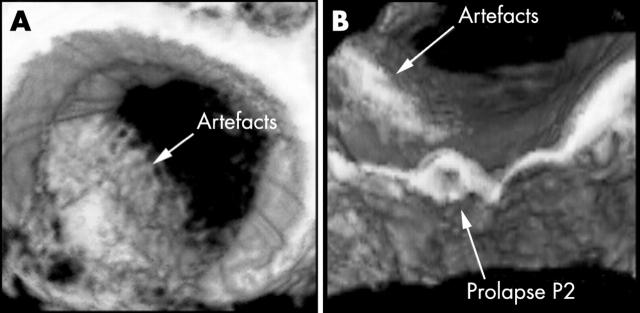
The development of this systematic method of 3D reconstruction was motivated by our experience that the commonly used en-face view (the so called surgeon’s eye view) was inadequate for assessment in many cases. This was confirmed by the fact that in 50% of en-face reconstructions we were unable to identify the pathology accurately. The principal reason for this was the presence of artefacts caused by reverberations in the left atrium, either from surrounding structures or from suboptimal probe contact. In order to visualise the course of a flail segment the cut plane of the en-face view must be of a sufficient height above the mitral annulus. This increases the chance of encountering artefacts. The use of longitudinal cut planes overcomes this problem (see example in fig 4).
Figure 4.
Example showing how the longitudinal plane allows better visualisation of the prolapse of P2 underneath the artefacts that obscure the pathology in the en-face view. P2, middle scallop of the posterior leaflets of the mitral valve.
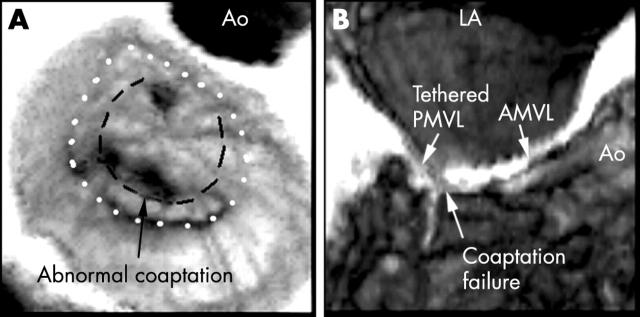
Recognition scores were significantly better for the longitudinal views, regardless of the type or location of pathology. In all but three patients we found that these views provided all the information required for clarification of the primary mechanism of mitral regurgitation. The incremental value of longitudinal reconstructions is demonstrated best by an example of ischaemic regurgitation where this view is superior in demonstrating pathology despite a very clear en-face view (fig 5). Ventricular views of the mitral valve have also been advocated by some investigators,25 but in our experience this view offers no additional information.
Figure 5.
An example showing that despite a good en-face view the pathology was best visualised in the longitudinal cut plane through A2–P2. The dotted line demarcates the mitral annulus and the dashed line shows the closure line between the two leaflets. The coaptation failure seen in the middle segment of the closure line was caused by tethering of P2. A2–P2, middle scallops of anterior and posterior leaflets; AMVL, anterior mitral valve leaflet; Ao, aorta; LA, left atrium; PMVL, posterior mitral valve leaflet.
Some studies have found en-face views alone to be adequate but have analysed relatively small numbers of non-rheumatic regurgitant valves.17,19 In contrast to our findings, some investigators have found that longitudinal views hindered the assessment of the whole valve because of excessive intensity of the reflected signals from near field structures.17 In our study, as the regions of interest in the longitudinal cut planes were solely the adjacent mitral segments, we were able to overcome this problem by concentrating on the optimisation of grey scale shading for these scallops in isolation. In fact the brightness of near field structures helped us obliterate artefacts by darkening the blood pool without jeopardising assessment of the adjacent valve scallops.
Reliance solely on en-face views wastes the most important benefit of 3D echocardiography—the ability to segment the dataset in any chosen plane. Our method of systematic analysis requires only a single acquisition in the mid to low oesophagus. The use of multiple longitudinal planes identified from the en-face views allows all segments of the valve to be scrutinised, particularly the commissural zones which may otherwise be difficult to identify.
It is of course possible to obtain similar longitudinal cuts on standard 2D multiplane imaging in some patients, and in many cases experienced TOE operators are able to build up a mental 3D image of the mitral valve from the conventional 2D images. However, in complex valves it is often very difficult to be confident about the position of the imaging plane along the closure line of the valve and whether that plane cuts through the leaflets perpendicular to the closure line or at an angle. These factors are of great importance in identifying the precise functional abnormality of the valve and hence its potential reparability. The primary advantage of 3D imaging is that the longitudinal planes can be positioned exactly perpendicular to the mitral closure line at any set point between the commissures. It is then possible to identify abnormalities of the leaflets precisely with reference to the positions of the commissures and the divisions between the scallops of the posterior leaflet and the corresponding zones of the anterior leaflet. In our view, this greatly facilitates the systematic assessment of complex valves, including those with multiple flail segments, perforations, and commissural abnormalities.
The evaluation of 20 randomly selected studies by an inexperienced observer showed closer agreement in the detection of pathology from longitudinal views than en-face views. Three dimensional reconstruction might reduce the long learning curve involved in the interpretation of complex mitral pathology.
In our study there was no significant difference between acquisitions under sedation and anaesthesia in terms of recognition of segments or detection of pathology. This is encouraging as 17% of acquisitions under sedation in our institution were unsuccessful. In patients who are unable to tolerate the 3D imaging under sedation, the acquisitions can be achieved in the anaesthetic room with great success and little inconvenience. In this latter setting we opted for acquisitions under apnoea because in our experience the quality of en-face reconstructions during positive pressure ventilation was inferior owing to the presence of respiratory artefacts. We made only a single attempt at 3D image acquisition, whether the TOE was performed under sedation or anaesthesia. Repeat attempts may have increased our chances of “perfect” en-face reconstructions, but in our opinion this would not have reflected the possible use of this method in routine clinical practice.
General anaesthesia reduced the acquisition time because apnoea abolished respiratory gating requirements. The similar acquisition times for sinus rhythm and atrial fibrillation are likely to reflect the small numbers of patients in fibrillation. Our 3D reconstruction times are in line with other studies20 and allow the preparation of volume rendered mitral valve reconstructions for review by the surgeons just before institution of cardiopulmonary bypass.20,21 This aside, we would still recommend a TOE study under sedation for quantification of mitral regurgitation, as this can be underestimated under general anaesthesia.32–34
We used findings at surgery as the gold standard against which to judge 2D and 3D echocardiography. The inherent problem in this approach is that the surgeon assesses an immobile valve in an empty heart whereas the echocardiographer assesses a dynamic valve in a beating heart. In the absence of a true gold standard imaging technique, there is no practical alternative. Both surgeons involved in this study had subspecialist experience in mitral repair surgery and analysed all valves to a set protocol using the techniques developed by Carpentier.13
The software that we used in this study was unable to acquire and analyse 3D colour flow data. Experimentally, 3D colour flow has been used to calculate regurgitant flow and may have advantages over conventional methods.35 Further advances in 3D software may allow the superimposition of 3D colour flow data onto a grey scale image in such a way that it might aid the identification of complex valve pathology.
We believe that this simple and reproducible method of analysis of the 3D dataset, combined with standard 2D assessment, allows optimal morphological assessment of the mitral valve, particularly when complex abnormalities are present.
REFERENCES
- 1.Carabello BA, Crawford FA. Valvular heart disease. N Engl J Med 1997;337:32–41. [DOI] [PubMed] [Google Scholar]
- 2.Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996;335:1417–23. [DOI] [PubMed] [Google Scholar]
- 3.Delahaye JP, Gare JP, Viguier E, et al. Natural history of severe mitral regurgitation. Eur Heart J 1991;12 (suppl B) :5–9. [DOI] [PubMed] [Google Scholar]
- 4.Enriquez-Sarano M , Tajik AJ, Schaff HV, et al. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 1994;90:830–7. [DOI] [PubMed] [Google Scholar]
- 5.Enriquez-Sarano M , Tajik AJ, Schaff HV, et al. Echocardiographic predictors of left ventricular function after correction of mitral regurgitation: results and clinical implications. J Am Coll Cardiol 1994;24:1536–43. [DOI] [PubMed] [Google Scholar]
- 6.Crawford MH, Souchek J, Oprian CA, et al. Determinants of survival and left ventricular performance after mitral valve replacement. Circulation 1990;81:1173–81. [DOI] [PubMed] [Google Scholar]
- 7.Rosen SE, Borer JS, Hochreiter C, et al. Natural history of the asymptomatic/minimally symptomatic patient with severe MR due to mitral valve prolapse and normal right and left ventricular performance. Am J Cardiol 1994;74:374–80. [DOI] [PubMed] [Google Scholar]
- 8.Kay GL, Aoki A, Zubiate P, et al. Probability of valve repair for pure mitral regurgitation. J Thorac Cardiovasc Surg 1994;108:871–9. [PubMed] [Google Scholar]
- 9.Marwick TH, Stewart WJ, Currie PJ, et al. Mechanisms of failure of mitral valve repair: an echocardiographic study. Am Heart J 1991;122:149–56. [DOI] [PubMed] [Google Scholar]
- 10.Hellemans IM, Pieper EG, Ravelli ACJ, et al. Prediction of surgical strategy in mitral valve regurgitation based on echocardiography. Am J Cardiol 1997;79:334–8. [DOI] [PubMed] [Google Scholar]
- 11.Ling LH, Enriquez-Sarano M, Seward JB, et al. Early surgery in patients with mitral regurgitation due to flail leaflets: a long-term outcome study. Circulation 1997;96:1819–25. [DOI] [PubMed] [Google Scholar]
- 12.Bonow RO, Carabello B, de Leon AC, et al. Guidelines for the management of patients with valvular heart disease: executive summary. Circulation 1998;98:1949–84. [DOI] [PubMed] [Google Scholar]
- 13.Carpentier A , Relland J, Deloche A, et al. Conservative management of the prolapsed mitral valve. Ann Thorac Surg 1978;26:294–302. [DOI] [PubMed] [Google Scholar]
- 14.Duran CG, Pomar JL, Revuelta JM, et al. Conservation operation for mitral insufficiency: critical analysis supported by postoperative haemodynamic studies of 72 patients. J Thorac Cardiovasc Surg 1980;79:326–37. [PubMed] [Google Scholar]
- 15.Chen Q , Nosir YFM, Vletter WB, et al. Accurate assessment of mitral valve area in patients with mitral stenosis by three-dimensional echocardiography. J Am Soc Echocardiogr 1997;10:133–40. [DOI] [PubMed] [Google Scholar]
- 16.Chen TO, Wang XF, Zheng LH, et al. Three-dimensional transesophageal echocardiography in the diagnosis of mitral valve prolapse. Am Heart J 1994;128:1218–24. [DOI] [PubMed] [Google Scholar]
- 17.Salustri A , Becker AE, Van Herwerden L, et al. Three-dimensional echocardiography of normal and pathologic mitral valve: a comparison with two-dimensional transesophageal echocardiography. J Am Coll Cardiol 1996;27:1502–10. [DOI] [PubMed] [Google Scholar]
- 18.Hozumi T , Yoshikawa J, Yoshida K, et al. Assessment of flail mitral leaflets by dynamic three-dimensional echocardiographic imaging. Am J Cardiol 1997;79:223–5. [DOI] [PubMed] [Google Scholar]
- 19.Yao J , Masani ND, Cao Q, et al. Clinical application of transthoracic volume-rendered three-dimensional echocardiography in the assessment of mitral regurgitation. Am J Cardiol 1998;82:189–96. [DOI] [PubMed] [Google Scholar]
- 20.Chauvel C , Bogino E, Clerc P, et al. Usefulness of three-dimensional echocardiography for the evaluation of mitral valve prolapse: an intraoperative study. J Heart Valve Dis 2000;9:341–9. [PubMed] [Google Scholar]
- 21.Abraham TP, Warner JG, Kon ND, et al. Feasibility, accuracy and incremental value of intraoperative three-dimensional transesophageal echocardiography in valve surgery. Am J Cardiol 1997;80:1577–82. [DOI] [PubMed] [Google Scholar]
- 22.Pandian NG, Nanda NC, Schwartz SL, et al. Three-dimensional and four-dimensional transesophageal echocardiographic imaging of the heart and aorta in humans using a computed tomographic imaging probe. Echocardiography 1992;9:677–87. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz SL, Cao QL, Azevedo J, et al. Simulation of intraoperative visualisation of cardiac structures and study of dynamic surgical anatomy with real-time three-dimensional echocardiography. Am J Cardiol 1994;73:501–7. [DOI] [PubMed] [Google Scholar]
- 24.Pandian NG, Roelandt JRTC, Nanda NC, et al. Dynamic three-dimensional echocardiography. Methods and clinical potential. Echocardiography 1994;11:237–59. [DOI] [PubMed] [Google Scholar]
- 25.Cheng TO, Xie MX, Wang XF, et al. Evaluation of mitral valve prolapse by four-dimensional echocardiography. Am Heart J 1997;133:120–9. [DOI] [PubMed] [Google Scholar]
- 26.Rivera JM, Vandervoort PM, Morris E, et al. Visual assessment of valvular regurgitation: comparison with quantitative Doppler measurements. J Am Soc Echocardiogr 1994;7:480–7. [DOI] [PubMed] [Google Scholar]
- 27.Pearson AC, Vrain J, Mrosek D, et al. Color Doppler echocardiographic evaluation of patients with a flail mitral leaflet. J Am Coll Cardiol 1990;16:232–9. [DOI] [PubMed] [Google Scholar]
- 28.Baxley WA, Kennedy JW, Field B, et al. Haemodynamics in ruptured chordae tendinae and chronic mitral regurgitation. Circulation 1973;48:1288–94. [DOI] [PubMed] [Google Scholar]
- 29.Pieper EP, Hellemans IM, Hamer HPM, et al. Value of systolic pulmonary venous flow reversal and color Doppler jet measurements assessed with transesophageal echocardiography in recognizing severe pure mitral regurgitation. Am J Cardiol 1996;78:444–50. [DOI] [PubMed] [Google Scholar]
- 30.Carpentier AF, Lessana A, Relland J, et al. The “physio-ring”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg 1995;60:1177–86. [DOI] [PubMed] [Google Scholar]
- 31.Kumar N , Kumar M, Duran C. A revised terminology for describing surgical findings of the mitral valve. J Heart Valve Dis 1995;4:70–7. [PubMed] [Google Scholar]
- 32.Pieper EP, Hellemans IM, Hamer HP, et al. Biplane transesophageal color-flow Doppler imaging in assessment of mitral regurgitation: influence of haemodynamic circumstances and mechanism of regurgitation. J Cardiothorac Vasc Anaesth 1996;10:748–55. [DOI] [PubMed] [Google Scholar]
- 33.Lishan A , Farzan F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischaemic mitral regurgitation? Circulation 2001;104:I68. [DOI] [PubMed] [Google Scholar]
- 34.Bach D , Deeb GM, Bolling SF. Accuracy of intraoperative transesophageal echocardiography for estimating the severity of functional mitral regurgitation. Am J Cardiol 1995;76:508–12. [DOI] [PubMed] [Google Scholar]
- 35.Rusk RA, Li XN, Mori Y, et al. Direct quantification of transmitral flow volume with dynamic 3-dimensional digital color Doppler: a validation study in an animal model. J Am Soc Echocardiogr 2002;15:55–62. [DOI] [PubMed] [Google Scholar]