Abstract
Four patients with nicorandil induced ulceration are described, and the literature on the subject is reviewed. Nicorandil induced ulcers are very painful and distressing for patients. Clinically they appear as large, deep, persistent ulcers that have punched out edges. They are poorly responsive to topical steroids and usually require alteration of nicorandil treatment. The ulceration tends to occur at high doses of nicorandil and all four cases reported here were on doses of 40 mg per day or greater. In these situations reduction of nicorandil dose may be sufficient to promote ulcer healing and prevent further recurrence. However, nicorandil induced ulcers have been reported at doses as low as 10 mg daily and complete cessation of nicorandil may be required.
Keywords: nicorandil, oral mucosa, ulceration
Nicorandil, a nicotinamide ester, is a potassium channel activator used in the prevention and long term treatment of angina pectoris.1 It is the only licensed drug in its class. It is a novel drug that acts as an arteriolar vasodilator, but also as a venodilator because of its nitrate moiety. It thus reduces both cardiac preload and afterload. It is not currently a first line agent in the management of angina but it is sometimes used in combination with other antianginal drugs in refractory stable and unstable angina.2 The drug has been used in Japan for over a decade and has more recently been introduced in Europe. Initial reports from France at the end of the last decade suggested a link between oral ulceration and the use of nicorandil,3–10 and more recent reports from the UK have also demonstrated a link.11–14 However, to date, no reports have appeared in the cardiology literature in the English language and there is a need to heighten awareness among cardiologists of the distressing nature of nicorandil induced oral ulceration. This paper presents four cases of nicorandil induced oral ulceration and reviews the literature on the subject.
CASE REPORTS
Case 1
A 72 year old man was referred to the oral medicine clinic by a dermatologist in relation to oral ulceration. The patient reported that he had developed large ulcers of 2–3 months’ duration over the previous 12 months. He had hypertension, ischaemic heart disease, benign prostatic hypertrophy, and arthritis and had undergone coronary artery bypass graft surgery (CABG) 24 years previously. His medications included nicorandil 40 mg twice daily, bisoprolol, amlodipine, losartan, frusemide, esomeprazole, tamsulosin, and glyceryl trinitrate transdermally. Nicorandil had been commenced two years previously and the dose gradually increased to the current dose of 40 mg twice daily. He had been prescribed triamcinolone acetonide 0.1% paste and benzydamine hydrochloride mouthwash for his oral ulceration.
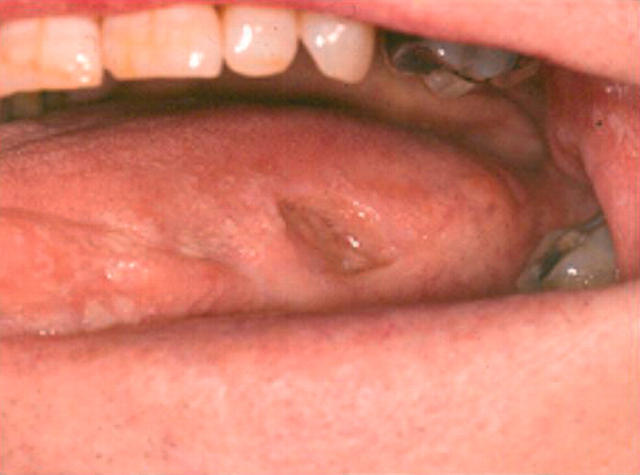
On examination, he had a round deep punched out ulcer on the left buccal mucosa measuring 15 mm in diameter. He had a similar ulcer measuring 10 mm by 7 mm on the left lateral border of his tongue (fig 1). There were also several smaller ulcers on the dorsum of his tongue. An incisional biopsy of lesional and paralesional tissue was carried out in the left buccal mucosa. Tissue was sent for histopathological and direct immunofluoresence studies. The histopathologist reported non-specific ulceration and there were no positive findings on direct immunofluoresence.
Figure 1.
Case 1: large painful ulcer on left lateral border of tongue, demonstrating the typical punched out appearance of nicorandil induced oral ulcers.
The patient was commenced on betamethasone 0.05% mouthwashes and the referring dermatologist was informed of the likely association between his oral ulceration and nicorandil. The nicorandil was not stopped while awaiting consultation with his cardiologist about adjusting his medication. Unfortunately the patient had a myocardial infarction shortly after this and died from renal failure 10 days later.
Case 2
A 54 year old man attended the accident and emergency department of the dental hospital complaining of painful oral ulceration of 10 weeks’ duration. He was diagnosed with angina in 1997 and had undergone CABG in 1998. He was a non-smoker. His medications included nicorandil 40 mg twice daily, bisoprolol, amlodipine, trimetazidine dihydrochloride, aspirin, atorvastatin, omeprazole, nimesulide, glyceryl trinitrate transdermally, and temazepam nocte. He used glyceryl trinitrate spray as required. His nicorandil dose had been increased from 30 mg to 40 mg twice daily nine months earlier.
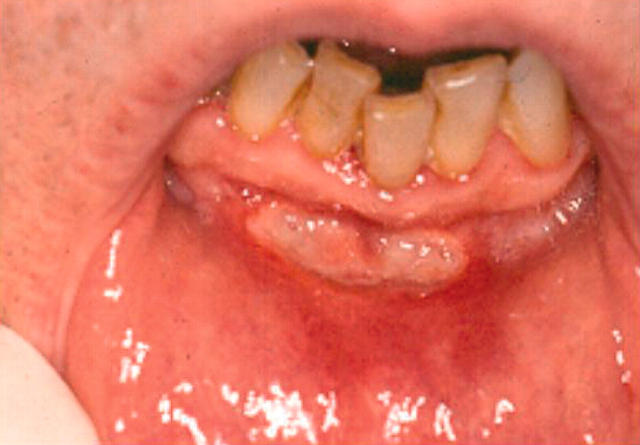
Clinical examination showed a large deep oval ulcer on the left lateral border of the tongue measuring 25 mm by 10 mm and a deep linear ulcer in the lower labial sulcus measuring 10 mm by 6 mm (fig 2). Both ulcers were surrounded by an erythematous flare. There was a strong clinical suspicion that the ulceration was caused by nicorandil. He was prescribed topical corticosteroid preparations, including betamethasone 0.05% mouth and triamcinolone acetonide 0.1% paste. His cardiologist, having been informed of the likely connection between nicorandil and his oral ulceration, discontinued the nicorandil. This was followed by rapid healing of the oral ulcers. The patient remained well with no exacerbation of his anginal symptoms or recurrence of oral ulceration.
Figure 2.
Case 2: ulcer of 10 weeks’ duration involving the lower labial sulcus.
Case 3
An 81 year old lady was referred to the oral medicine clinic by her general medical practitioner. She gave a five month history of painful oral ulceration occurring on her palate and buccal mucosa. She had ischaemic heart disease and had undergone CABG in 1991, following which she developed a deep venous thrombosis and pulmonary embolism. She also suffered from peripheral vascular disease and cervical spondylosis. She was a non-smoker. Her medications included nicorandil 30 mg twice daily, lisinopril, bisoprolol, aspirin, omeprazole, and glyceryl trinitrate transdermally. She also took glyceryl trinitrate spray and diclofenac acid as required. She was first placed on nicorandil 10 mg twice daily in October 1999; this was increased to 20 mg twice daily in December 2000 and was further increased to 30 mg twice daily in March 2001. Her ulceration developed four weeks after this last dose increase.
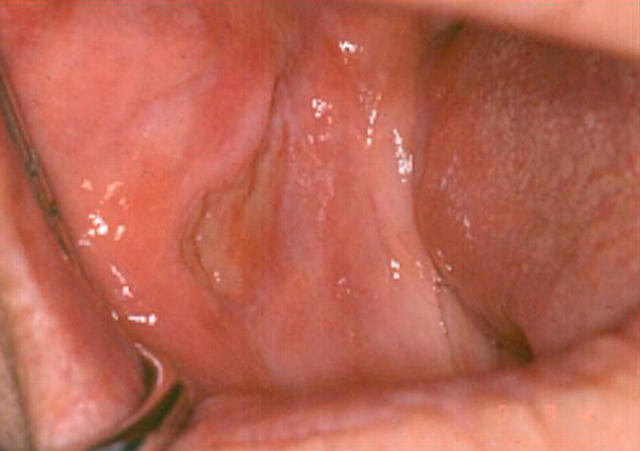
Clinical examination revealed the presence of a large deep ulcer measuring approximately 20 mm by 15 mm in the left buccal mucosa (fig 3). A course of 2.5 mg hydrocortisone pellets was prescribed for topical application to the oral ulcer. The clinical appearance was suggestive of a nicorandil induced oral ulcer and liaison with her cardiologist resulted in discontinuation of the nicorandil. On review six weeks later the ulceration had resolved. Fortunately, the patient’s angina remained well controlled and there were no further episodes of oral ulceration.
Figure 3.
Case 3: large ulcer involving the left buccal mucosa.
Case 4
A 75 year old woman was referred to the oral medicine clinic by her general medical practitioner in relation to a large ulcer on the ventral surface of her tongue of three weeks’ duration. She suffered with unstable angina and had a myocardial infarction and CABG 10 years previously. She also had a pulmonary embolus 10 years previously and had ventricular ectopic beats. Her medications included nicorandil 20 mg twice daily, isosorbide mononitrate, diltiazem, amiodarone, atorvastatin, digoxin, warfarin, frusemide, and glyceryl trinitrate spray as required. She had been prescribed 2.5 mg hydrocortisone pellets and triamcinolone acetonide 0.1% paste but these had not resulted in an improvement in her symptoms.
On intra-oral examination there was a deep linear ulcer on the left side of the ventral surface of the tongue. There was no associated lymphadenopathy. The clinical appearance of the ulcer was strongly suggestive of nicorandil induced ulceration. Betamethasone 0.05% mouthwash six times daily was prescribed and this resulted in resolution of the ulcer. She had further ulcer recurrences, but the ulcers were aborted by the early use of betamethasone mouthwash and her cardiologist was reluctant to discontinue nicorandil. She suffered an exacerbation in her ulceration approximately three months after her nicorandil dose was increased to 50 mg daily and she re-presented with a large painful ulcer measuring 3 cm in diameter on the left buccal mucosa. This eventually resolved with the continued use of betamethasone mouthwashes. Unfortunately, shortly after this the patient died suddenly.
DISCUSSION
Nicorandil is, in general, a well tolerated drug. Its well recognised side effects include headache, flushing, nausea, dizziness, hypotension, and tachycardia. A total of 49 cases of nicorandil associated oral ulceration have been reported worldwide since 19973–16 (table 1), but many of these have appeared in the French literature. Those cases in the English literature have been published in general or oral journals and this paper is an attempt to increase awareness among the cardiology community of this distressing side effect of nicorandil.
Table 1.
Reports of nicorandil induced oral ulceration, together with the number of cases and the countries of origin
| Authors | Number of cases | Country |
| Boulinguez et al 19973 | 3 | France |
| Reichart et al 19974 | 2 | France |
| Agbo-Godeau et al 19985 | 16 | France |
| Cribier et al 19986 | 7 | France |
| Desruelles et al 19987 | 2 | France |
| Roussel et al 19988 | 1 | France |
| Vincent et al 199910 | 1 | France |
| Shotts et al 199911 | 3 | UK |
| Gupta et al 200012 | 1 | UK |
| Haas et al 200015 | 1 | France |
| Laveau et al 200016 | 1 | France |
| Scully et al 200113 | 9 | UK/Portugal |
| Pemberton et al 200114 | 2 | UK |
The oral ulceration associated with nicorandil is characterised by large deep persistent ulcers. The ulcers cause considerable discomfort and interfere greatly with eating and speaking and thus have a negative impact on quality of life and may result in weight loss.6 The ulcers tend to be poorly responsive to topical steroid treatment, but heal rapidly, usually without scarring, following discontinuation of the drug.5,13 Marquart-Elbaz et al9 carried out a prospective study to investigate the prevalence of nicorandil induced ulceration and found oral ulceration in 5% of 100 patients taking nicorandil and no ulceration in a group of 100 patients taking other antianginal drugs.
However, it is important to point out that not all oral ulceration in patients taking nicorandil can be attributed to the drug. Oral cancer often presents as a deep, persistent oral ulcer and therefore it is essential to exclude this. In any case where the diagnosis is in doubt the patient should be referred to an oral physician and a biopsy carried out, if appropriate.
There has been confusion in the literature about the nature of these lesions, with many authors describing them as major or giant aphthous ulcers. However, drugs have been only rarely implicated in aphthous ulceration, those drugs implicated including non-steroidal anti-inflammatory drugs.17 Drugs have been more commonly implicated in other forms of oral ulceration, in particular lichenoid drug reactions and erythema multiforme. A recent study has reported the visual assessment of photographs of nicorandil associated lesions by 60 dermatologists and the outcome was that most regarded the ulcers as non-aphthous in appearance.18 This would concur with our view that these lesions are not aphthous in nature. They have some similarities with major aphthous ulcers in that they are large and have a protracted healing time, but they tend to lack the yellow base and erythematous halo that are usually associated with aphthous ulcers and they have a punched out appearance which is not seen in aphthous ulceration. Thus we would favour the term nicorandil induced ulcers.
Most cases of nicorandil induced ulceration that have had biopsies of their lesions carried out demonstrated non-specific ulceration5,6,11,13 as seen in the single case that was biopsied by us, although there have been isolated cases of eosinophilic ulceration reported.6,9,16
While it is clear that the lesions heal rapidly following cessation of nicorandil, the severity of ischaemic heart disease may mitigate against this. All the patients described here had severe ischaemic heart disease and were on maximal antianginal therapy. In the two cases where nicorandil was stopped, anginal symptoms had stabilised but the cessation of nicorandil was not without risk. Fortunately, their angina remains well controlled. Nicorandil associated ulceration generally occurs at high doses, with patients usually not experiencing ulceration at low doses, but only when the dose is increased to 40–60 mg daily.4,12 In these incidences, it may be sufficient to reduce the dose of the drug and complete discontinuation may not be necessary. However, nicorandil associated ulceration has been reported with a dose as low as 10 mg/day.16
There has been a suggestion by Watson et al19 that nicorandil may be associated with anal ulceration. They described five cases of anal ulceration that were deep and had a punched out appearance, similar to that seen in the mouth. All patients were taking nicorandil 60 mg daily, with the exception of one who was taking 20 mg daily. The ulceration healed within a few weeks of stopping the drug in all cases. Histological samples were taken from all cases, all of which showed granulation tissue associated with acute inflammation.
The mechanism by which nicorandil produces oral ulceration is unclear. There may be a direct local toxic effect of nicorandil on the oral mucosa. Nicorandil or a metabolite may be secreted in saliva and similarly produce a toxic effect. However, faecal excretion of nicorandil is known to be low, and yet it has been implicated in anal ulceration. Alternatively the oral ulcers could be a manifestation of a hypersensitivity reaction to nicorandil.
In conclusion, nicorandil can be associated with intractable oral ulceration that can be extremely distressing for the patient. It is poorly responsive to treatment and usually only responds to nicorandil cessation or a reduction in its dose. As with all cases of persistent oral ulceration, it is important to ensure that the ulceration is nicorandil induced and is not neoplastic, and therefore referral to an oral physician should be made where the diagnosis is in doubt.
REFERENCES
- 1.Frampton J , Buckley M, Fitton A. Nicorandil. A review of its pharmacology and therapeutic efficacy in angina pectoris. Drugs 1992;44:625–55. [DOI] [PubMed] [Google Scholar]
- 2.The IONA study group. Effect of nicorandil on coronary events in patients with stable angina: the impact of nicorandil in angina (IONA) randomised trial. Lancet 2002;359:1269–75. [DOI] [PubMed] [Google Scholar]
- 3.Boulinguez S , Bedane C, Bouyssou-Gauthier M, et al. Giant buccal aphthosis caused by nicornadil. Presse Med 1997;26:558. [PubMed] [Google Scholar]
- 4.Reichart S , Antunes A, Trechot P, et al. Major aphthous stomatitis induced by nicorandil. Eur J Dermatol 1997;7:132–3. [Google Scholar]
- 5.Agbo-Godeau S , Joly P, Lauret P, et al. Association of major aphthous ulcers and nicorandil. Lancet 1998;352:1598–9. [DOI] [PubMed] [Google Scholar]
- 6.Cribier B , Marquart-Elbaz C, Lipsker D, et al. Chronic buccal ulceration induced by nicorandil. Br J Dermatol 1998;138:372–3. [DOI] [PubMed] [Google Scholar]
- 7.Desruelles F , Bahadoran P, Lacour J, et al. Giant oral aphthous ulcers induced by nicorandil. Br J Dermatol 1998;138:712–3. [DOI] [PubMed] [Google Scholar]
- 8.Roussel S , Courville P, Peron J, et al. Oral aphthae induced by nicorandil. Anatamopathologic aspects. Rev Stomatol Chir Maxillofac 1998;99:207–9. [PubMed] [Google Scholar]
- 9.Marquart-Elbaz C , Lipsker D, Grosshans E, et al. Oral ulcers induced by nicorandil: prevalence and clinico-pathological aspects. Ann Dermatol Venereol 1999;126:587–90. [PubMed] [Google Scholar]
- 10.Vincent S , Andreani V, Janin-Manificat L, et al. Case report of a patient treated with nicorandil who developed buccal aphthosis. Therapie 1999;54:260–1. [PubMed] [Google Scholar]
- 11.Shotts R , Scully C, Avery C, et al. Nicorandil-induced severe oral ulceration. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Endod 1999;87:706–7. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A , Morris G. Major aphthous ulcers induced by nicorandil. Age Ageing 2000;29:372–3. [DOI] [PubMed] [Google Scholar]
- 13.Scully C , Crighton C, Felix D, et al. Nicorandil can induce severe oral ulceration. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Endod 2001;91:189–93. [DOI] [PubMed] [Google Scholar]
- 14.Pemberton M , Theaker E, Sloan P. Nicorandil and oral ulceration. Oral Surg, Oral Med, Oral Pathol, Oral Radiol, Endod 2001;92:2. [DOI] [PubMed] [Google Scholar]
- 15.Haas C , Dendoune F, Persoz M, et al. Nicorandil-induced giant lingual aphthosis in a patient with Behcet’s disease. Presse Med 2000;29:2092–3. [PubMed] [Google Scholar]
- 16.Laveau F , Chapuis H, Dandurand M, et al. Eosinophilic ulceration of the tongue. Ann Dermatol Venereol 2000;127:716–8. [PubMed] [Google Scholar]
- 17.Healy C , Thornhill M. An association between recurrent oro-genital ulceration and non-steroidal anti-inflammatory drugs. J Oral Pathol Med 1995;24:46–8. [DOI] [PubMed] [Google Scholar]
- 18.Boulinguez S , Sommet A, Bedane C, et al. Oral nicorandil-induced lesions are not aphthous ulcers. J Oral Pathol Med 2003;32:482–5. [DOI] [PubMed] [Google Scholar]
- 19.Watson A , Al Ozairi O, Fraser A, et al. Nicorandil-associated anal ulceration. Lancet 2002;360:546–7. [DOI] [PubMed] [Google Scholar]