Abstract
Objective: To determine the performance of a new NT-proBNP assay in comparison with brain natriuretic peptide (BNP) in identifying left ventricular systolic dysfunction (LVSD) in randomly selected community populations.
Methods: Blood samples were taken prospectively in the community from 591 randomly sampled individuals over the age of 45 years, stratified for age and socioeconomic status and divided into four cohorts (general population; clinically diagnosed heart failure; patients on diuretics; and patients deemed at high risk of heart failure). Definite heart failure (left ventricular ejection fraction (LVEF) < 40%) was identified in 33 people. Samples were handled as though in routine clinical practice. The laboratories undertaking the assays were blinded.
Results: Using NT-proBNP to diagnose LVEF < 40% in the general population, a level of > 40 pmol/l had 80% sensitivity, 73% specificity, 5% positive predictive value (PPV), 100% negative predictive value (NPV), and an area under the receiver-operator characteristic curve (AUC) of 76% (95% confidence interval (CI) 46% to 100%). For BNP to diagnose LVSD, a cut off level of > 33 pmol/l had 80% sensitivity, 88% specificity, 10% PPV, 100% NPV, and AUC of 88% (95% CI 75% to 100%). Similar NPVs were found for patients randomly screened from the three other populations.
Conclusions: Both NT-proBNP and BNP have value in diagnosing LVSD in a community setting, with similar sensitivities and specificities. Using a high cut off for positivity will confirm the diagnosis of LVSD but will miss cases. At lower cut off values, positive results will require cardiac imaging to confirm LVSD.
Keywords: natriuretic peptides, left ventricular systolic dysfunction, heart failure
Left ventricular systolic dysfunction (LVSD) is an increasingly common disorder,1 with a prevalence of around 2%.2,3 It has a poor prognosis4 and is associated with a reduced quality of life,5 especially where it is the cause of heart failure. As outcomes in heart failure and LVSD are linked to the stage of disease, early and accurate diagnosis is needed to guide appropriate management.
Even in symptomatic patients, the diagnosis of LVSD is not possible on clinical grounds alone, as objective evidence of cardiac dysfunction is required6,7; this is usually obtained from echocardiographic studies. A reliable diagnosis of LVSD is important because several treatments, especially the use of angiotensin converting enzyme (ACE) inhibitors8 and β blockers,9 improve symptoms, quality of life, and disease prognosis, and reduce health care use and costs. However, most patients with symptomatic LVSD are presumed to have LVSD on clinical grounds alone, without further investigation.10 Furthermore, early diagnosis may also be important in asymptomatic LVSD, as treatment can delay or reverse disease progression and the onset of heart failure.11,12 Diagnostic tools may therefore need to encompass screening strategies13,14 as well as case identification in symptomatic patients.
LVSD is usually investigated by echocardiography, which provides a semi-objective assessment of ventricular and valve function. However, access to echocardiography is limited in many health care systems,11 especially in primary care, and therefore the validity of a clinical diagnosis of LVSD in primary care is often poor when patients are assessed against objective criteria.15 The ECG has been advocated as an easy non-invasive method for diagnosing LVSD, as a normal recording will, in most cases, exclude left ventricular dysfunction.16,17 However, many non-specialists18 are unreliable at assessing ECG changes.
Potential new diagnostic aids are natriuretic peptide assays. Brain natriuretic peptide (BNP) is a 32 amino acid biologically active peptide, cleaved from the 108 amino acid proBNP, released from the cardiac ventricles in response to chamber stretch. BNP has vasodilator and natriuretic properties. The second remnant following cleavage, N-terminal proBNP (NT-proBNP), is a 76 amino acid with no known biological function.
BNP showed a high sensitivity and modest specificity in confirming LVSD in 1653 adults (aged 25–75 years) screened in an area of high cardiovascular disease19 and in 155 elderly patients over 75 years screened in primary care.20 NT-proBNP has been less investigated, but the assay used in this study reported a sensitivity of 100%, a specificity of 70%, a positive predictive value (PPV) of 7%, a negative predictive value (NPV) of 100%, and an area under the receiver-operator characteristic (ROC) curve of 92% (95% confidence interval (CI) 82% to 100%) in the diagnosis of heart failure in the ECHOES (echocardiographic Heart of England screening) cohort.21 In this paper we report complementary original data on the value of the NT-proBNP assay in the diagnosis of LVSD in various community populations and compare the data with the performance of the most commonly used BNP assay.
METHODS
This was a prospective substudy of the ECHOES study of LVSD and heart failure prevalence.2 The main study was carried out in 16 randomly selected primary care practice populations in England following stratification for age and socioeconomic status. Patients were randomly selected from each of four discrete population cohorts, identified in each practice from computerised practice registers, comprising:
randomly sampled patients from those aged 45 years and older (general population screen)
patients with a clinical (unvalidated) diagnostic label of heart failure
patients prescribed diuretic drugs
patients at high risk of heart failure (history of previous myocardial infarction, angina, hypertension, or diabetes).
This natriuretic peptide substudy was conducted in the last four practices, containing the final 607 consecutively screened subjects randomly selected from the four target groups (307 in the general population, 103 with an existing clinical diagnosis of heart failure, 87 taking diuretics, and 134 at high risk of heart failure, with some sampled from more than one cohort). These practices were representative of the socioeconomic spread of patients achieved by the initial stratification.
All patients were screened by history, New York Heart Association (NYHA) functional class, clinical examination, quality of life (short form 36 item (SF-36) health status questionnaire), spirometry, resting 12 lead ECG, and echocardiography, including Doppler studies. LVSD was defined as an ejection fraction under 40% by an area–length method from the apical four chamber view.22
Blood samples for natriuretic peptide assay were taken from the right antecubital fossa of consenting subjects after five minutes of supine rest into 5 ml K+EDTA tubes. Blood was kept at room temperature for up to 24 hours before transport to a local laboratory for centrifugation and freezing of plasma to −20°C. Once a week, the frozen samples were collected for central storage at −70°C. Assays of plasma BNP (Shinogi, Japan) and NT-proBNP (Roche Diagnostics, Germany) were subsequently done at a central laboratory, blinded to the screening assessments. The BNP immunoradiometric assay (IRMA) requires several operator dependent steps, including sample extraction, with total incubation of 18 to 22 hours. The NT-proBNP immunoassay (enzyme linked immunosorbent assay (ELISA)) requires three operator dependent steps and total incubation of under two hours.
Analysis
The diagnostic performance of BNP was assessed using ROC curves,23 formed by plotting sensitivity on the y axis and 1−specificity on the x axis for all possible cut off values of each diagnostic test. The overall discriminatory ability of the tests is demonstrated by the area under the curve (AUC).24 In addition to the AUCs, we identified the cut off value from the general population which maximised sensitivity without much loss of specificity. Likelihood ratios were also calculated, as a measure of the extent to which the pretest odds are altered by the test results (values > 1 increase the odds, values < 1 decrease the odds). The cut off values were then applied to the groups with a previous history of heart failure, diuretic use, and at high risk of heart failure. The resulting positive predictive values (PPV), negative predictive values (NPV), likelihood ratios (LR), and confidence intervals (CI) are presented.
The Hanley and McNeil method24 was used to compare the ROC curves of the two assays. Statistical analyses were carried out using SPSS and Microsoft EXCEL.
RESULTS
Of 607 eligible subjects, 591 (97%) consented to supply a blood sample. The basic demographics of the population screened are listed in table 1 and overall performance characteristics of the two assays and of the ECG in table 2.
Table 1.
Characteristics of total and left ventricular dysfunction (LVSD) populations
| Characteristic | Total (n = 591) | LVSD (n = 33) |
| Age (years) | 65.8 (10.7) | 71.1 (9.0) |
| Sex (male)* | 316 (53.5) | 25 (75.8) |
| Height (m) | 1.7 (0.1) | 1.7 (0.1) |
| Weight (kg) | 75.4 (15.3) | 76.9 (15.6) |
| BMI (kg/m2) | 27.2 (4.7) | 26.8 (3.9) |
| Ethnicity: white* | 573 (97.0) | 33 (100) |
| Ever smoked* | 351 (59.4) | 21 (63.6) |
| History of MI* | 87 (14.7) | 7 (21.2) |
| History of angina* | 127 (21.5) | 3 (9.1) |
| History of hypertension* | 232 (39.3) | 0 (0) |
| History of diabetes* | 68 (11.5) | 1 (3) |
| Heart rate (beats/min) | 70.9 (13.4) | 72.1 (12.6) |
| Systolic BP (mm Hg) | 153.5 (22.6) | 139.2 (24.3) |
| Diastolic BP (mm Hg) | 84.4 (11.5) | 79.1 (11.1) |
| FEV1 (litre) | 2.21 (0.81) | 1.90 (0.68) |
| FVC (litre) | 2.63 (0.93) | 2.30 (0.76) |
Values are mean (SD) or *n (%).
Total population data reproduced with permission from Hobbs et al, BMJ 2002;324:1498–502.
BMI, body mass index; BP, blood pressure; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; MI, myocardial infarction.
Table 2.
Area under receiver operator characteristic (ROC) curve, sensitivity, specificity, positive and negative predictive values, and likelihood ratios for brain natriuretic peptide and NT-proBNP in the diagnosis of left ventricular systolic dysfunction
| Cohort sampled | Test | AUC (95% CI) | Cut off value (pmol/l) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) |
| General population over 45 (n = 307) | NT-proBNP | 0.76 (0.46 to 1.0) | 40 | 80% (28 to 100) | 73% (68 to 78) | 5% (1 to 12) | 100% (98 to 100) | 2.95 (1.83 to 4.74) | 0.27 (0.05 to 1.59) |
| BNP | 0.88 (0.75 to 1.0) | 33 | 80% (28 to 100) | 88% (84 to 92) | 10% (3 to 24) | 100% (98 to 100) | 6.71 (3.93 to 11.46) | 0.23 (0.04 to 1.31) | |
| Patients with previous HF diagnosis (n = 103) | NT-proBNP | 0.70 (0.58 to 0.81) | 40 | 100% (87 to 100) | 18% (11 to 28) | 24% (15 to 34) | 100% (82 to 100) | 1.22 (1.10 to 1.36) | 0 (0 to 1.38) |
| BNP | 0.70 (0.59 to 0.82) | 33 | 71% (48 to 89) | 52% (41 to 64) | 28% (17 to 42) | 88% (75 to 95) | 1.50 (1.05 to 2.14) | 0.54 (0.23 to 1.29) | |
| Patients on diuretics (n = 87) | NT-proBNP | 0.81 (0.60 to 1.0) | 40 | 86% (42 to 100) | 40% (29 to 52) | 11% (4 to 23) | 97% (84 to 100) | 1.43 (1.01 to 2.03) | 0.36 (0.06 to 2.24) |
| BNP | 0.80 (0.60 to 0.99) | 33 | 86% (42 to 100) | 65% (54 to 75) | 18% (7 to 35) | 98% (90 to 100) | 2.44 (1.60 to 3.75) | 0.22 (0.04 to 1.36) | |
| Patients at high risk for HF (n = 133) | NT-proBNP | 0.73 (0.62 to 0.84) | 40 | 100% (74 to 100) | 46% (37 to 56) | 13% (7 to 23) | 100% (95 to 100) | 1.86 (1.58 to 2.20) | 0 (0 to 1.01) |
| BNP | 0.70 (0.57 to 0.82) | 33 | 50% (19 to 81) | 67% (58 to 75) | 11% (4 to 24) | 94% (87 to 98) | 1.51 (0.77 to 2.95) | 0.75 (0.33 to 1.70) |
AUC, area under the curve; CI, confidence interval; HF, heart failure.
Definite left ventricular systolic dysfunction, defined as a validated ejection fraction of less than 40%, was detected in 33 people from the four diagnostic groups: five from the general population, 21 from those with an existing heart failure diagnosis, seven from those on diuretic treatment, and 10 in the high risk group (10 patients were in more than one group).
Diagnosis of left ventricular systolic dysfunction using NT-proBNP
Using NT-proBNP to diagnose LVSD (LVEF < 40%), in the general population (fig 1), a cut off level of > 40 pmol/l had a sensitivity of 80% (95% CI 28% to 100%), a specificity of 73% (95% CI 68% to 78%), a PPV of 5% (95% CI 1% to 12%), an NPV of 100% (95% CI 98% to 100%), an LR+ of 2.9 (95% CI1.8 to 4.7), an LR− of 0.3 (95% CI 0.05 to 1.6), and an AUC of 0.76 (95% CI 0.46–1.0).
Figure 1.
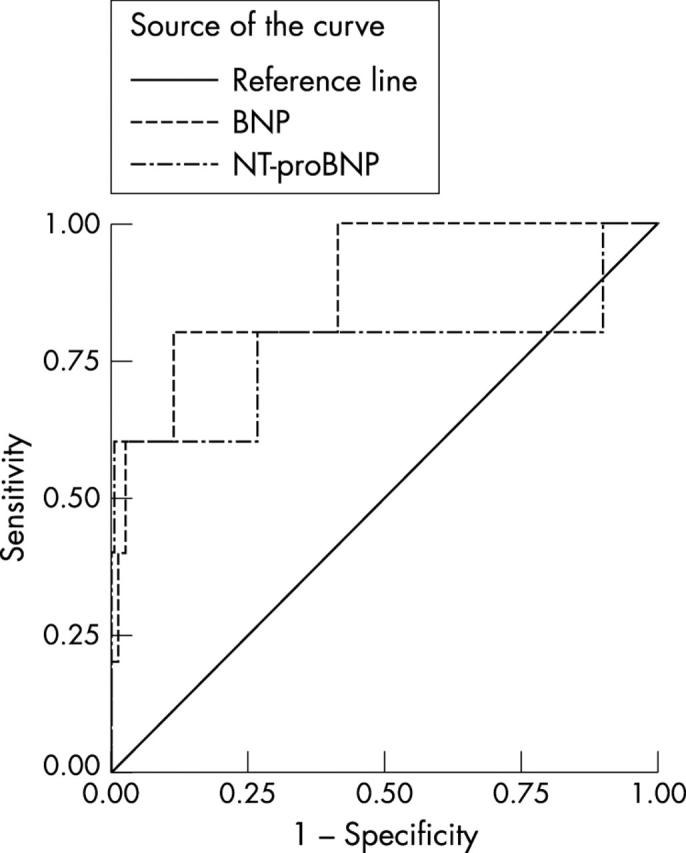
Receiver-operator characteristic (ROC) curves for brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) in the diagnosis of definite left ventricular systolic dysfunction (left ventricular ejection fraction < 40%) in the general population.
Diagnosis of left ventricular systolic dysfunction using brain natriuretic peptides
For BNP, a cut off of > 33 pmol/l had a sensitivity of 80% (95% CI 28% to 100%), a specificity of 88% (95% CI 84% to 92%), a PPV of 10% (95% CI 3% to 24%), an NPV of 100% (95% CI 98% to 100%), an LR+ of 6.7 (95% CI 3.9 to 11.5), an LR− of 0.2 (95% CI 0.04 to 1.3), and an AUC of 0.88 (95% CI 0.75 to 1.0) (fig 2). Similar performance characteristics were found using the same cut off values for the other population cohorts (figs 3 and 4), the NPVs, ranging from 88% to 100% and LR− from 0 to 0.75. The differences between the AUCs of the two natriuretic peptides were not significant in the diagnosis of LVSD.
Figure 2.
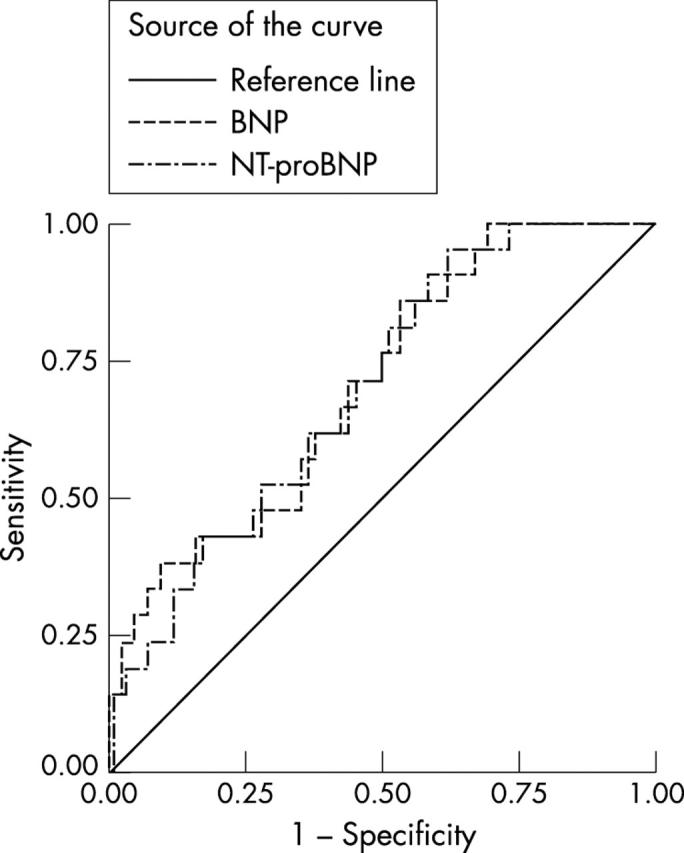
Receiver-operator characteristic (ROC) curves for brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) in the diagnosis of definite left ventricular systolic dysfunction (left ventricular failure) in the population with an established clinical diagnosis of heart failure.
Figure 3.
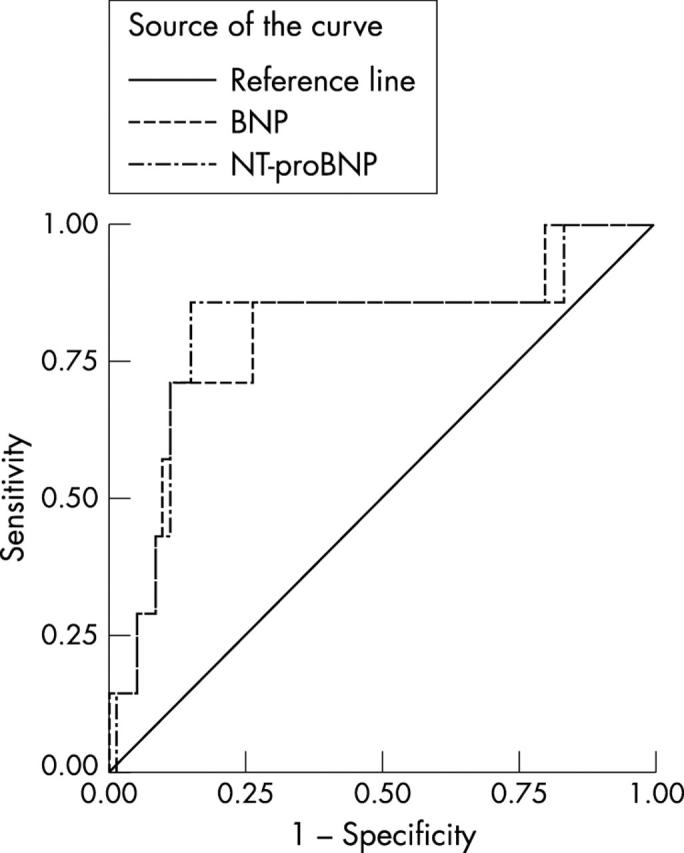
Receiver-operator characteristic (ROC) curves for brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) in the diagnosis of definite left ventricular systolic dysfunction (left ventricular failure) in the population on diuretics.
Figure 4.
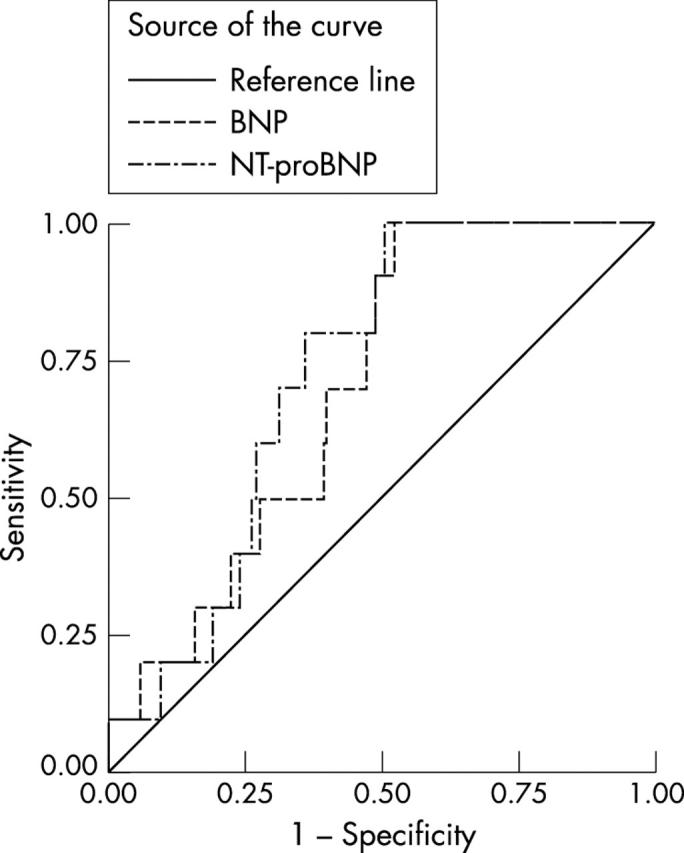
Receiver-operator characteristic (ROC) curves for brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) in the diagnosis of definite left ventricular systolic dysfunction (left ventricular failure) in the high risk population.
DISCUSSION
In contrast to earlier studies of BNP assay performance in select patient groups, we believe these are the first data to compare the performance characteristics of both BNP and NT-proBNP assays in the diagnosis of LVSD, to compare the performance of the assays with that of ECGs, and to examine the assays in two populations: a large generalisable series of randomly selected adults with a validated LVSD diagnosis; and a comparator normative population, randomly selected from the same populations as the cases. The assays were also used under the same conditions and constraints as pertain to routine clinical practice. This “real world” study also sought to minimise the main confounding variables of diagnostic studies—namely, spectrum bias (the performance characteristics are presented separately for the different risk populations), verification bias, treatment paradox (any treatment effect will have diluted the size of the test performance), and reference standard error.
Both BNP and NT-proBNP assays were shown to be of value in diagnosing LVSD within a representative community based general adult population and also within at risk populations, with AUCs of at least 0.7.
The natriuretic assays were handled as they would be in everyday practice of primary care, namely collection by nurses and storage at room temperature for hours, so they should be more generalisable than earlier studies with specialised in-hospital handling of samples, including immediate chilled centrifugation and rapid freezing of samples to −70°C. Furthermore, the assays appear to be useful despite the influence of treatment on the peptide values,25 which could reduce the value of the assays in all but newly presenting patients.26
These data extend the positive conclusions of our earlier study reporting the performance characteristics of the NT-proBNP and BNP assays in the diagnosis of heart failure. With the high negative predictive values of both the BNP assays reported in this study, LVSD can effectively be ruled out at assay concentrations below our suggested cut off values. Such test characteristics suggest that the most appropriate use of the assays in routine practice would be as exclusion tests, where positive values would result in further cardiac investigation, including echocardiography. Choosing a higher cut off would become diagnostic of LVSD, but at the expense of missing cases.
The use of the assays as a triage test leading to echocardiography, as proposed above, would be useful in clinical practice because cardiac imaging would stage the degree of LVSD as well as confirming the diagnosis, thus guiding treatment intensity. Such a “pre-screening” use of the tests is analogous to cervical cytology in determining referral for colposcopy in cervical cancer screening. However, this interpretation of our data on the use of natriuretic peptide assays in LVSD diagnosis should be formally tested by cost–effectiveness trials of the assays in symptomatic and high risk screening strategies.
Acknowledgments
The ECHOES study was funded by the NHS R&D cardiovascular disease and stroke programme (FDRH and MKD were joint principal investigators). The costs of collecting and handling the assays were supported by an NHS Regional R&D grant, and the assays were donated by Roche Diagnostic Solutions.
FDRH and MKD were joint principal investigators and grant holders on the main study, with FDRH grant holder of this substudy. FDRH led the design and execution of the study and analysis. RCD conducted the majority of the clinical assessments and reporting, and made a major contribution to analysis. JEK and RH contributed to study design and analysis and overall study management. AKR performed statistical analysis. All authors contributed to drafts of the paper.
Competing interests: FDRH is a member of the European Society of Cardiology (ESC) Working Party on Heart Failure, is Chair of the British Primary Care Cardiovascular Society (PCCS), and Treasurer of the British Society for Heart Failure. MKD is Chairman of the British Society for Heart Failure. FDRH and MKD have received travel sponsorship and honoraria from a number of biotechnology and pharmaceutical companies with cardiovascular products for plenary talks and attendance at major cardiology scientific congresses and conferences.
Abbreviations
AUC, area under receiver-operator characteristic curve
BNP, brain natriuretic peptide
ECHOES, echocardiographic Heart of England screening study
LR, likelihood ratio
LVEF, left ventricular ejection fraction
LVSD, left ventricular systolic dysfunction
NPV, negative predictive value
PPV, positive predictive value
REFERENCES
- 1.Bonneaux L , Barendregt JJ, Meeter K, et al. Estimating clinical morbidity due to ischemic heart disease and congestive heart failure: the future rise of heart failure. Am J Public Health 1994;84:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies MK, Hobbs FDR, Davis RC, et al. Prevalence of left ventricular systolic dysfunction and heart failure in the general population: main findings from the ECHOES (echocardiographic Heart of England screening) study. Lancet 2001;358:439–45. [DOI] [PubMed] [Google Scholar]
- 3.McDonagh TA, Morrison CE, Lawrence A, et al. Symptomatic and asymptomatic left ventricular systolic dysfunction in an urban population. Lancet 1997;350:829–33. [DOI] [PubMed] [Google Scholar]
- 4.Ho KKL, Anderson KM, Kannel WB, et al. Survival after onset of congestive heart failure in Framingham heart study subjects. Circulation 1993;88:107–15. [DOI] [PubMed] [Google Scholar]
- 5.Davis R , Hobbs FDR, Kenkre JE, et al. Quality of life in heart failure, as measured by SF-36 health status questionnaire. Eur Heart J 1998;19:S639. [Google Scholar]
- 6. Task Force on Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis of heart failure. Eur Heart J 1995;16:741–51. [PubMed] [Google Scholar]
- 7. American College of Cardiology/American Heart Association. Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on evaluation and management of heart failure) Circulation 1995;92:2764–84. [DOI] [PubMed] [Google Scholar]
- 8.Garg R , Yusuf S. for the Collaborative Group on ACE Inhibitor Trials. Overview of randomised trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450–6. [PubMed] [Google Scholar]
- 9.CIBIS-II Investigators and Committees. The cardiac insufficiency bisoprolol study II. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 10.Hobbs FDR, Jones MI, Allan TF, et al. European survey of primary care physician perceptions on heart failure diagnosis and management (Euro-HF). Eur Heart J 2000;21:1877–87. [DOI] [PubMed] [Google Scholar]
- 11. The SOLVD investigators. Effect of Enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992;327:685–91. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Brunwald E, Moy LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. N Engl J Med 1992;327:669–77. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs FDR. Primary care physicians: champions of or an impediment to optimal care of the patient with heart failure? Eur J Heart Failure 1999;1:1:11–15. [DOI] [PubMed] [Google Scholar]
- 14.Dargie HJ, McMurray JJV. Diagnosis and management of heart failure. BMJ 1994;308:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remes J , Miettinen H, Reunanen A, et al. Validity of clinical diagnosis of heart failure in primary health care. Eur Heart J 1991;12:315–21. [DOI] [PubMed] [Google Scholar]
- 16.Davie AP, Francis CM, Love MP, et al. Value of an electrocardiogram in identifying heart failure due to left ventricular systolic dysfunction. BMJ 1996;312:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The NETWORK Investigators. Clinical outcome with enalapril in symptomatic chronic heart failure; a dose comparison. Eur Heart J 1998;19:481–9. [DOI] [PubMed] [Google Scholar]
- 18.Sur DK, Kaye L, Mikus M, et al. Accuracy of electrocardiogram reading by family practice residents. Fam Med 2000;32:315–19. [PubMed] [Google Scholar]
- 19.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left ventricular systolic dysfunction. Lancet 1998;351:9–13. [DOI] [PubMed] [Google Scholar]
- 20.Smith H , Pickering RM, Struthers A, et al. Biochemical diagnosis of ventricular dysfunction in elderly patients in general practice: observational study. BMJ 2000;320:906–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs FDR, Davis RC, Roalfe AK, et al. Reliability of N-terminal pro-brain natriuretic peptide assay in diagnosis of heart failure: cohort study in representative and high risk community populations. BMJ 2002;324:1498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hewlett Packard Co. HP acoustic quantification user’s guide. PN77021-98160/1/2/4. Andover, Massachusetts: Hewlett Packard Corporation,.
- 23.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–77. [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–43. [DOI] [PubMed] [Google Scholar]
- 25.Murdoch DR, McDonagh TA, Bryne J. Titration of vasodilator therapy in chronic heart failure according to plasma brain natriuretic peptide concentration: randomised comparison of haemodynamic and neuroendocrine effects of tailored versus empirical therapy. Am Heart J 1999;138:1126–32. [DOI] [PubMed] [Google Scholar]
- 26.Struthers AD. The diagnosis of heart failure. Heart 2000;84:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


