Abstract
γ-Aminobutyric acid type B (GABAB) receptors mediate the metabotropic actions of the inhibitory neurotransmitter GABA. These seven-transmembrane receptors are known to signal primarily through activation of G proteins to modulate the action of ion channels or second messengers. The functional GABAB receptor is made up of a heterodimer consisting of two subunits, GABAB-R1 and GABAB-R2, which interact via coiled-coil domains in their C-terminal tails. By using a yeast two-hybrid approach, we have identified direct interactions between the C-terminal tails of GABAB-R1 and GABAB-R2 with two related transcription factors, CREB2 (ATF4) and ATFx. In primary neuronal cultures as well in recombinant Chinese hamster ovary cells expressing GABAB receptors, CREB2 is localized within the cytoplasm as well as the nucleus. Activation of the GABAB receptor by the specific agonist baclofen leads to a marked translocation and accumulation of CREB2 from the cytoplasm into the nucleus. We demonstrate that receptor stimulation results in activation of transcription from a CREB2 responsive reporter gene. Such a signaling mechanism is unique among Family C G protein-coupled receptors and, in the case of the GABAB receptor and CREB2, may play a role in long-term changes in the nervous system.
GABA (γ-aminobutyric acid) is the major inhibitory neurotransmitter activating both ionotrophic GABAA and GABAC receptors as well as metabotropic GABAB receptors (1). GABAB receptors were originally identified pharmacologically (2) and couple through G proteins to Ca2+ or K+ channels. Despite being recognized many years ago, the molecular nature of the GABAB receptor has been elucidated only recently (3–8). The initial GABAB “receptor,” GABAB-R1, was expression cloned by using a high-affinity antagonist and showed homology to Family C G protein-coupled receptors (GPCRs), such as metabotropic glutamate receptors, with a characteristically large extracellular N-terminal domain as well as a seven-transmembrane topology (3). However, when expressed recombinantly, GABAB-R1 failed to reproduce the expected agonist affinities (3, 5) and was not expressed at the cell surface (5, 9), suggesting that a component was lacking from GABAB-R1 for reconstitution of the functional receptor. Subsequent studies confirmed that a second distinct but related GPCR, GABAB-R2, heterodimerizes with GABAB-R1 to create the GABAB-receptor (4–8). Yeast two-hybrid (YTH) studies showed that the two receptors interact through a coiled-coil domain within their respective C-terminal tails (5, 7, 10), and in situ hybridization analysis showed colocalization of the two receptors (4, 5, 7, 10). The expressed heterodimer reproduced expected agonist pharmacology, and in the presence of GABAB-R2, GABAB-R1 was trafficked to the cell surface. Moreover, effector couplings to K+ and Ca2+ channels have now been demonstrated by using the heterodimeric receptor (4, 6, 11, 12).
We previously used a YTH screen to identify GABAB-R2 as an interacting protein partner to GABAB-R1 (5). Besides GABAB-R2, we identified a second class of proteins that were highly represented in the screen and gave a robust interaction with the C terminus of GABAB-R1. These proteins are the transcription factors CREB2 (cAMP responsive element binding protein-2; ATF4) and ATFx, which are both members of the ATF/CREB family of transcription factors (13), characterized by C-terminal basic leucine zipper (bZIP) DNA-binding domains and N-terminal transcriptional domains. CREB2 is a ubiquitously expressed 351-amino acid protein with a typical C-terminal bZIP domain and an N terminus containing consensus mitogen activated protein kinase (MAPK) phosphorylation sites but lacking protein kinase A and protein kinase C phosphorylation sites (14). CREB2 both activates (15) and represses (14, 16) transcription and can form dimers with other transcription factors including those in the CCAAT/enhancer-binding protein family (17). ATFx was identified originally as a partial mouse cDNA showing high homology to CREB2 (18). Human ATFx has been cloned recently as a partial cDNA of 122 amino acids with 57% identity to CREB2 over the bZIP region but a distinct N terminus. Like CREB2, ATFx is ubiquitously expressed and can repress cAMP-dependent protein kinase-induced transcription (19).
Here we present an analysis of the GABAB receptor and associated transcription factors. We show that CREB2 and ATFx interact with both GABAB receptor subunits by YTH and establish that the interaction of CREB2 with GABAB-R1 can be detected in vivo in brain extracts. Moreover, we demonstrate that CREB2, when expressed either in Chinese hamster ovary (CHO) cells together with the GABAB receptor or in primary neuronal cultures, is distributed within the cytosol as well as the nucleus. Activation of the GABAB receptor in these cells triggers the relocation of cytosolic CREB2 to the nucleus, leading to a transcriptional response. Such a mechanism of transcriptional activation by direct association of a transcription factor is a unique observation amongst Family C GPCRs and represents a previously uncharacterized mechanism of signal transduction.
Materials and Methods
Materials.
Baclofen, CGP54626, and PD98059 were purchased from Tocris Neuramin (Bristol, U.K.). Pertussis toxin (PTX) was obtained from Sigma. Primary antibodies used were CREB2 antisera, a rabbit polyclonal (Sc-20; Santa Cruz Biotechnology); GABAB-R1a antisera, a sheep polyclonal (raised against peptide N-DGSRVHLLYK-C, The Babraham Institute); GABAB-R2 antisera, a rabbit polyclonal (raised against peptide N-VPPSFRVMVSGL-C, The Babraham Institute); splicing factor Sc-35 antiserum, a mouse monoclonal (Sigma); FLAG M2 antisera, a mouse monoclonal antisera (Sigma); and glial fibrillary acidic protein (GFAP) antisera, a mouse monoclonal-CY3 conjugate (Sigma).
Constructs.
GABAB-R1 and GABAB-R2 C termini were generated by PCR and cloned in frame with GAL4BD in pYTH9 and pYTH16 for YTH studies (20); GAL4AD fusions for CREB2 and ATFx activating transcription factor were expressed in pACT2 (CLONTECH), either isolated directly from human brain cDNA library or constructed directly by using PCR. Full-length tagged myc-GABAB-R1b and HA-GABAB-R2 were constructed as described (5). Expression of full-length CREB2 was mediated by pcDNA3.1 (Invitrogen). CREB2 was N-terminally tagged by addition of the FLAG octapeptide N-DYKDDDDK-C in pcDNA3.1 by PCR. Likewise, enhanced green fluorescent protein (eGFP; CLONTECH) was fused to the N terminus of CREB2 within pcDNA3.1. The chloramphenicol acetyltransferase (CAT) assay reporter construct p9000 comprises the growth arrest and DNA damage (Gadd153) promoter (−788 to +21) fused to the CAT gene (21).
YTH Studies.
Yeast (Saccharomyces cerevisiae Y190) expressing either a GAL4-binding domain–GABAB-R1 C terminus fusion protein or a GAL4-binding domain–GABAB-R2 C terminus fusion protein were selected and transformed with a human brain Matchmaker cDNA library (HL4004AH, CLONTECH) to give a 3-fold representation of the library. Interacting clones were selected with 20 mM 3-amino-1,2,4-triazole (Sigma) followed by production of β-galactosidase as determined by a freeze-fracture assay. Plasmid DNA was recovered from yeast by using the Yeastmaker plasmid isolation kit (CLONTECH), and the resulting DNA was transformed into Escherichia coli before sequencing. Direct two-hybrid interactions were determined in Y190 cotransformed with pYTH16, containing the C terminus or C-terminal coiled-coil domain of GABAB-R1 or GABAB-R2 fused to GAL4BD, and pACT2, expressing CREB2 or ATFx as GAL4AD fusions. Fusion constructs were generated directly through PCR or were retrieved as isolates from the library screen. Levels of interaction were determined by β-galactosidase freeze-fracture assay.
Cell Biology.
HEK293T cells were maintained in DMEM plus 10% (vol/vol) FCS/2 mM glutamine, transfected with cDNA (3 μg total) by using Lipofectamine (Life Technologies, Paisley, Scotland), and harvested 48–72 h after transfection. Plasma membrane containing P2 particulate fractions (5) were prepared for binding studies.
CHO cells stably expressing GABAB-R1a and GABAB-R2 receptor subunits or ORL1 were maintained in DMEM/Ham's F-12 medium supplemented with 10% (vol/vol) FCS, 2 mM glutamine, 10 mg/ml G418, and 5 mg/ml hygromycin. DNA transfections were carried out by using Lipofectamine (Life Technologies).
Coimmunoprecipitations.
HEK293T cells were harvested and homogenized in lysis buffer [50 mM Tris⋅Cl/150 mM NaCl/1% Nonidet P40/0.5% (wt/vol) sodium deoxycholate, pH 7.5] supplemented with Complete protease inhibitor mixture (Roche Molecular Biochemicals). Precleared supernatant was collected by incubation with 50 μl of Protein A-agarose (Roche Molecular Biochemicals). Immunoprecipitation with either FLAG or Myc antisera was allowed to proceed for 1 h at 4°C, and capture of immune complexes was progressed overnight after the addition of 50 μl protein A agarose. Immune complexes were collected by microcentrifugation, released from protein A agarose, and analyzed by SDS/PAGE followed by immunoblotting.
In vivo coimmunoprecipitation experiments were performed by using rat brain homogenates from 6-week-old male Sprague-Dawley rats, prepared in 1% Triton X-100 as described (22). Protease inhibitors (Roche Molecular Biochemicals) were included throughout, and all procedures were performed at 4°C. Samples of solubilized brains (2 ml) were incubated with 100 μl of packed protein G agarose (Amersham Pharmacia) for 1 h at 4°C. The agarose was removed, and the supernatant incubated with 2 μg of either affinity-purified sheep anti-GABAB antibody or pooled normal sheep IgG for 3 h. Capture of immune complexes was progressed by using protein G agarose (25 μl of packed resin) for 2 h. Four washes of the resin were performed, and the material was eluted with SDS sample buffer (50 μl) by heating at 100°C for 2 min. The samples were analyzed on SDS/9% polyacrylamide. Immunoblotting for Creb2 was performed by using the Creb2 antibody at 2 μg/ml.
[35S]GTPγS Binding Assays.
[35S]GTPγS binding assays were performed at room temperature as described (23).
Confocal Microscopy.
CHO cells stably expressing receptors were plated directly on 22-mm sterile glass coverslips in 6-well dishes and transfected with pcDNA3-eGFP-CREB2 (0.05 μg per well). After transfection for 48 h, cells were viewed by Confocal Microscopy (Leica TCS NT, Deerfield, IL) at 37°C at 488-nm excitatory wavelength. Cells were pretreated with either PTX (50 ng/ml; 16 h) or CGP54626 (1 μM; 30 min) before addition of baclofen (100 μM). Cells were examined for over 1 h.
CHO-GABAB-R1/GABAB-R2 Cell Fractionation.
CHO cell cultures were fractionated by using standard procedures (24) in the presence of protease inhibitors (Roche Molecular Biochemicals), and cytoplasmic extracts were analyzed by immunoblotting.
CAT Reporter Gene Assays.
CHO cells stably expressing GABAB receptors were plated in T75 flasks, and each flask was transfected with pcDNA3-CREB2 (3 μg), p9000 (1 μg; Gadd153 promoter-CAT reporter gene; ref. 15), and pcDNA3 (1 μg). Cells were incubated in normal medium for 24 h and serum free media for a further 24 h. Next, cells were treated with baclofen (1 mM to 0.1 μM; 6 h). CAT levels were determined in cytoplasmic extracts by using a CAT ELISA kit (Roche Molecular Biochemicals).
For inhibitor studies, transfected cells were pretreated with either PTX (50 ng/ml; 16 h), CGP56426 (1 μM; 30 min), or PD98059 (50 μM; 30 min) before addition of baclofen (100 μM; 6 h). CAT levels were determined by ELISA.
Neuronal Cultures and Nuclear Fractionation.
Cortical hemispheres from E17 rat embryos were dissected, and primary cultures of rat cortical neurons were prepared (25). Cells were cultured for 4 weeks without frequent medium changes. On day 1 in vitro, the medium was replaced with fresh BNG medium [neurobasal medium (Gibco) containing 50 μg/ml gentamycin (26) and cytosine-β-d-arabinofuranose (Sigma) to inhibit glial growth (5 μM, days 1–7, 2.5 μM thereafter)]. Nuclear fractionation was conducted by using standard techniques (24), and the nuclear and cytoplasmic fractions were analyzed by Western blotting and quantified.
Fluorescence Immunohistochemistry.
Primary cultures of rat cortical neurons were grown on glass coverslips for 15–20 days and fixed in 4% (vol/vol) paraformaldehyde at room temperature for 5 min, washed with PBS (2 ml), and incubated with 50 mM Tris⋅HCl, pH 7.2/0.02% Triton X-100/0.1 M NaCl for 5 min. Slides were incubated with blocking solution for at least 15 min (Tris saline containing 1% BSA and 0.1% normal donkey serum) followed by primary antibodies (room temperature; 2 h). Slides were next washed three times with 2 ml of blocking solution and incubated with secondary antibodies (The Jackson Laboratory; 2 h at room temperature). Slides were washed as before, followed by a final wash with Tris saline before mounting on microscope slides in Vectashield (Vector Laboratories) and sealing with nail varnish. Slides were viewed on a fluorescence microscope (Leica Leitz DMRB), and images were captured by using a Xillix digital camera.
Coronal freezing microtome sections (30 μm) were blocked with normal serum and incubated sequentially overnight with GABABR1, GFAP, or CREB2 antisera. Secondary antibodies used were FITC-coupled donkey anti-sheep IgG (Jackson ImmunoResearch) and Texas Red-coupled goat anti-rabbit IgG (Vector Laboratories). Fluorescence was detected by using a laser scanning confocal microscope (Bio-Rad MRC1024).
Results
Identification of CREB2 and ATFx as Interacting Partners to the GABAB Receptor.
The C termini of the GABAB-R1 and GABAB-R2 were screened against a human brain cDNA library by using YTH (5). Besides the paired GABAB receptor subunits, we recovered two related transcription factors, CREB2 (ATF4) and ATFx, as interacting proteins. CREB2 and ATFx share 57% homology over their bZIP domains, and both repress protein kinase A-induced transcription (14, 16, 19). From the YTH screen, we cloned a 282-amino acid ORF for ATFx, which we believe to be full length. The N-terminal sequences show no homology to CREB2 but contain two consensus MAPK-phosphorylation sites and several proline-rich repeats of uncertain function.
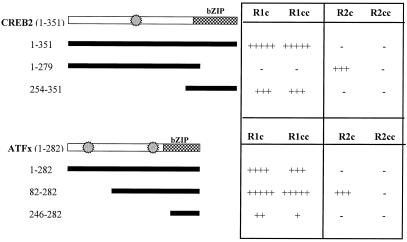
The interacting domains of both GABAB subunits with CREB2 and ATFx were defined further by using YTH (Fig. 1). CREB2 and ATFx both interact with the C termini of GABAB-R1 and GABAB-R2, with their bZIP domains responsible for their interactions with GABAB-R1 but not for their association with GABAB-R2. Full-length CREB2 and ATFx fail to interact with GABAB-R2; however, CREB2, lacking its bZIP domain, or ATFx, lacking its far N terminus, does strongly interact with GABAB-R2, suggesting some particular conformation requirement for association. The coiled-coil domain of GABAB-R1 specifically interacts with CREB2 and ATFx, but in contrast, the coiled-coil domain of GABAB-R2 does not associate with either CREB2 or ATFx as the complete C terminus, suggesting that the GABAB-R2 interaction is not via the coiled-coil domain. CREB2 and ATFx are unable to homodimerize and heterodimerize via their bZIP domains in the YTH assay (results not shown).
Figure 1.
Interactions between CREB2, ATFx, and GABAB receptor subunits, as determined by β-galactosidase activity in the YTH system. CREB2 and ATFx GAL4AD fusion protein and GABAB receptor C termini GAL4BD fusion proteins were coexpressed in the YTH system, and β-galactosidase activity was determined. The regions of the GABAB-R1 expressed were amino acids 854–961 (GABAB-R1a) and amino acids 877–928 for the coiled-coil (CC) domain. The amino acids expressed from the GABAB-R2 C terminus were 744–941 and 779–847 for the coiled-coil domain. The intensity of the resulting β-galactosidase activity is indicated by the number of + signs. The relative positions of the bZIP domain and the putative MAPK phosphorylation sites are shown as hatched boxes and star symbols, respectively.
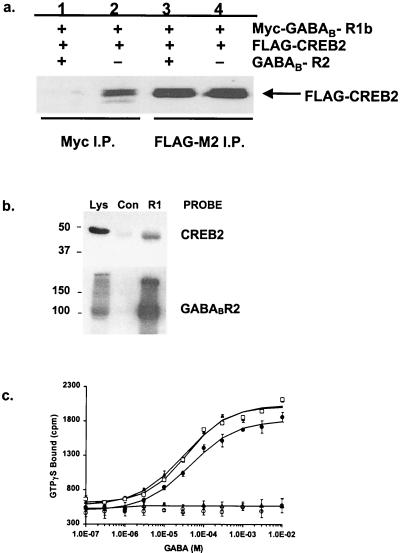
To demonstrate a direct interaction in mammalian cells, either Myc-epitope-tagged GABAB-R1b or HA-epitope-tagged GABAB-R2—both of which are known to co-immunoprecipitate (5)—was transiently expressed in HEK293T cells either alone or in combination with FLAG-epitope-tagged CREB2. Immunoprecipitation of GABAB-R1 with anti-Myc antisera led to detection of FLAG-CREB2 within immune complexes by using anti-FLAG antisera (Fig. 2a). In contrast, HA-GABAB-R2 did not coimmunoprecipitate with FLAG-CREB2 (data not shown), and this result mirrors that of the YTH. Therefore, the GABAB-R2–CREB2 interaction, which is only seen with smaller domains expressed in the YTH system, may involve some protein conformation masked within the full-length proteins. Therefore, the significance of the CREB2 and GABAB-R2 association, as identified by YTH, is unclear, because we cannot confirm the interaction in our mammalian immunoprecipitation assay.
Figure 2.
Interaction of GABAB-R1 and GABAB-R2 with the Transcription factor CREB2. (a) Myc-GABAB-R1b and Flag-CREB2 coimmunoprecipitation (I.P.) from HEK293T cells, transfected with Myc-GABAB-R1b and Flag-CREB2. GABAB-R1 and CREB2 were immunoprecipitated by 9E10 (Myc) antisera (lanes 1 and 2) or anti-Flag antisera (lanes 3 and 4), and the precipitates were immunoblotted with anti-Flag antisera. Cotransfection of cells with GABAB-R2 (lane 1 and 3) blocked coimmunoprecipitation of GABAB-R1 and CREB2. GABAB-R2 coexpression had no effect on the direct immunoprecipitation of FLAG-CREB2 with anti-Flag antisera (lanes 3 and 4). (b) GABAB-R1 and CREB2 coimmunoprecipitation from rat-brain homogenates. Immunoblots of total lysate (Lys), together with immunoprecipitates against affinity purified GABAB-R1 antisera (R1) or pooled Sheep normal IgG (Con), are shown. Lysates show the presence of GABAB-R1 and CREB2, whereas the GABAB-R1 antisera coprecipitated a strong CREB2 immunoreactive band at the expected size. (c) Coexpression of CREB2 with GABAB-R1 and GABAB-R2 receptors in HEK293T cells does not affect GABA-mediated stimulation of [35S]GTPγS-binding activity. GABA stimulation of [35S]GTPγS binding in cells transfected with GABAB-R1b and GABAB-R2 in the absence (□) and presence of either CREB2 (●) or eGFP-CREB2 (▴) or in combination with GABAB-R1b together with CREB2 (▵) or eGFP-CREB2 (○). The data shown are the means ± SD of triplicate measurements and are representative of three independent experiments.
We next examined whether coexpression of GABAB-R2 affects the interaction of GABAB-R1 with CREB2 (Fig. 2a). GABAB-R2 (1 μg of DNA) was transfected into HEK293T cells together with constant amounts of Myc-GABAB-R1 (1 μg) and FLAG-CREB2 (1 μg); interaction of Myc-GABAB-R1 and FLAG-CREB2 was studied by coimmunoprecipitation. Addition of GABAB-R2 prevented the interaction of GABAB-R1 with CREB2, suggesting that the binding of GABAB-R2 and CREB2 to GABAB-R1 is mutually exclusive. Therefore, either CREB2 does not bind to the GABAB heterodimer through the coiled-coil motif and dimer dissociation must occur for CREB2 to interact with GABAB-R1 or CREB2 interacts with the heterodimer in a different way than it does with the GABAB-R1 monomer. To confirm that the interaction between GABAB-R1 and CREB2 was of physiological relevance in vivo, we examined whether the proteins directly interact in rat brain. Immunoprecipitation of GABAB-R1 from detergent-solubilized rat brain membranes led to detection of CREB2 within immune complexes by using anti-CREB2 antisera (Fig. 2b). This coimmunoprecipitation confirms that the interaction between GABAB-R1 and CREB2 occurs in vivo.
Interaction with CREB2 Does Not Influence Receptor Function.
To examine whether coexpression of CREB2 affects receptor function, we used [35S]GTPγS binding to measure GABAB receptor activity. Coexpression of CREB2 with GABAB receptors in HEK293T cells did not affect GABA-mediated stimulation of [35S]GTPγS-binding activity (Fig. 2c), suggesting that CREB2 does not alter G protein activation by the GABAB receptor.
Subcellular Localization of CREB2.
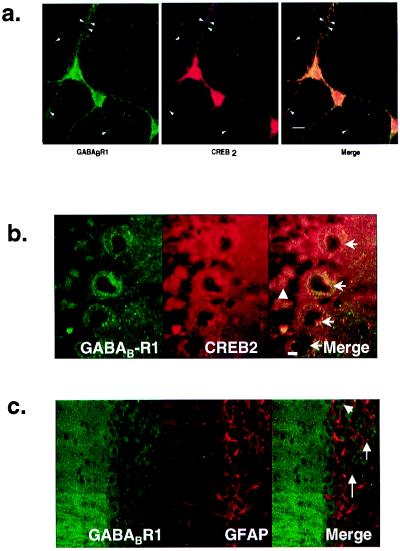
We next examined the locations of GABAB-R1 and CREB2 proteins both in rat primary cortical cultures and rat cerebellar slices by fluorescence immunohistochemistry (Fig. 3). Immunostaining of cortical cultures for CREB2 showed a range of nuclear staining for CREB2 with some nuclei well defined by CREB2 immunoreactivity, whereas others showed a weaker, more diffuse pattern of staining. In addition, CREB2 revealed a pronounced perinuclear staining and diffuse punctate staining throughout the cell soma and distal dendrites. GABAB-R1 immunoreactivity was found throughout the cell soma and dendrites, and there was a large degree of codistribution of the two proteins (Fig. 3a). In rat cerebellar slices, very high levels of GABAB-R1 immunoreactivity were observed, which likewise codistributed with CREB2 in the molecular layer as well as within Purkinje and granule cells (Fig. 3b). In contrast, GFAP, despite being highly expressed in the cerebellum, did not colocalize with GABAB-R1 (Fig. 3c).
Figure 3.
Codistribution of GABAB-R1 and CREB2 in neurons. (a) Codistribution of GABAB-R1 and CREB2 in rat primary cortical cultures. Fixed rat-cortical neurons (E17) were stained with primary antibodies for GABAB-R1 and CREB2. Images were viewed for GABAB-R1 (green) and CREB2 (red), and the images were merged. [bar = 10 μm]. Arrowheads indicate areas of distinct codistribution for the two proteins in the distal dendrites. (b) Codistribution of GABAB-R1 and CREB2 in rat-cerebellar cortex. Fluorescence was detected by confocal microscopy for GABABR1 (green) and CREB2 (red), and the images were merged. Arrows indicate Purkinje cells, and the arrowhead shows granule cells. [bar = 15 μm]. (c) Distinct non-codistribution of GABAB-R1 and GFAP in rat-cerebellar cortex. Fluorescence was detected by confocal microscopy for GABABR1 (green) and GFAP (red), and the images were merged. [bar = 15 μm].
GABAB Receptors Alter CREB2 Localization.
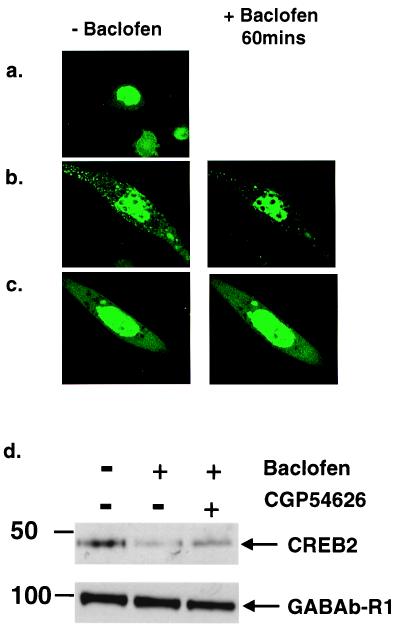
To visualize directly CREB2 within cells, we constructed a fusion protein of eGFP fused to the N terminus of CREB2, which we transfected into CHO cells stably expressing either ORL1 or the GABAB receptors (Fig. 4 a–c). Transfection of eGFP-CREB2 in CHO cells expressing ORL1, which couples to the same effectors as GABAB, resulted in specific localization of eGFP-CREB2 to the nucleus. In contrast, transfection of eGFP-CREB2 into CHO cells stably expressing GABAB receptors resulted in a diffuse/punctate pattern of fluorescence throughout the cytoplasm as well as strong nuclear fluorescence. Next, we treated cells with the GABAB receptor agonist, baclofen (100 μM). In three independent experiments, a dramatic loss of the eGFP-CREB2 signal was observed from the cytoplasm, with maximal effects occurring after 60 min (Fig. 4b). In several cells, nuclear fluorescence appeared to increase at the same time, suggesting translocation of eGFP-CREB2 to the nucleus. Coincubation of the cells with baclofen plus the selective GABAB antagonist CGP54626 (1 μM; ref. 27) inhibited the baclofen-stimulated loss of eGFP-CREB2 fluorescence from the cytoplasm, confirming that this response is receptor mediated.
Figure 4.
GABAB receptor activation alters CREB2 localization in CHO-GABAB cells. (a) CHO-ORL1 cells show bright nuclear localization of eGFP-CREB2. (b) CHO-GABAB cells show diffuse and punctate cytoplasmic fluorescence for eGFP-CREB2 as well as bright nuclear fluorescence. Treatment with 100 μM baclofen significantly decreases cytoplasmic eGFP-CREB2. (c) GABAB antagonist CGP54626 (1 μM) blocks the effects of baclofen (100 μM). (d) Immunoblots of cytoplasmic extracts prepared from CHO-GABAB cells, transfected with CREB2, show decreased levels of CREB2 in the cytoplasm after baclofen treatment (100 μM), whereas CGP54626 (1 μM) partially blocked the decrease in CREB2 levels. Cytoplasmic levels of GABAB-R1 remained constant.
To quantify the baclofen-induced CREB2 translocation in these cells, we carried out immunoblot detection of CREB2 on cytosolic fractions from control and baclofen-treated cells (Fig. 4d). CHO cells stably expressing GABAB receptors expressed CREB2 in the cytosolic fraction. Treatment of the cultures with baclofen (100 μM; 60 min) resulted in a significant reduction in CREB2 within the cytoplasmic fraction. This effect was greatly diminished by cotreatment with antagonist CGP54626 (1 μM).
Similar studies were conducted in rat primary cortical cultures visualizing endogenous CREB2 with a specific polyclonal CREB2 antiserum. Treatment of cultures with 20 μM baclofen resulted in an increase in the punctate nature of CREB2-IR within dendrites, with a concomitant loss of CREB2-IR from the soma and dendrites over a time course of 60 min. Counting five or six random fields in three independent experiments yielded 8% of CREB2-IR neurons showing discrete nuclear staining before baclofen treatment as opposed to 27% after baclofen treatment. This represents a 3.5-fold increase in CREB2 brightly staining nuclei over 1 h with baclofen treatment.
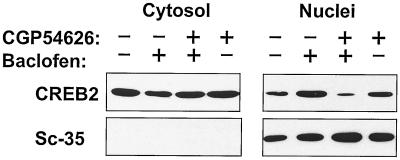
To quantify further the response, we carried out immunoblot detection of CREB2 on nuclear fractions from control and baclofen-treated cortical cultures (Fig. 5). Cortical neurons expressed CREB2 in both nuclear and cytosolic fractions; treatment of the cultures with baclofen (20 μM; 60 min) resulted in an approximately 3-fold increment in CREB2 within nuclear extracts [n = 4; mean nuclear increase = 3.3 ± 1.3 (SD)]. This effect was again abolished by simultaneous treatment with CGP54626 (1 μM).
Figure 5.
Baclofen increases CREB2 levels in the nuclei in primary rat cortical cultures. Treatment of cortical cultures with baclofen (20 μM) gave a 3-fold increase in CREB2 immunoreactivity in nuclear extracts in contrast to cytoplasmic extracts, as determined by Western blotting. The nuclear localization of CREB2 was blocked by the GABAB receptor antagonist CGP54626 (1 μM). Sc-35 localized entirely to the nuclear extract and showed no alteration in levels with baclofen treatment. Means of four independent experiments reveal a 3.3 ± 1.3 (SD) increase in nuclear CREB-IR after baclofen treatment.
Transcriptional Activation by CREB2 in Response to Baclofen.
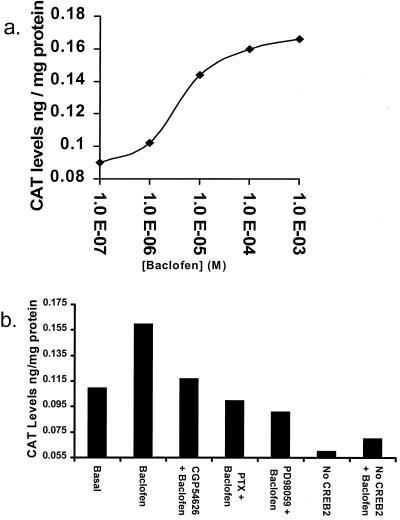
CREB2 is a specific transcription factor regulating expression of the Gadd153 gene in response to stress. The Gadd153 promoter contains a CCAAT/enhancer-binding protein-ATF consensus site, which binds CREB2 to activate Gadd153 gene transcription (21). To study baclofen-mediated effects on Gadd153 gene transcription through CREB2, we transiently transfected a reporter gene consisting of the Gadd153 promoter linked to CAT (21) together with pcDNA3-CREB2 (3 μg) into CHO cells stably expressing the GABAB receptor (Fig. 6). Baclofen gave a clear dose-dependent increase in CAT levels in the CHO-GABAB cells, with an EC50 of 4 μM. Activation by baclofen (100 μM) was abolished by addition of CGP54626 (1 μM), again suggesting that activation is receptor mediated. Moreover, increases in CAT expression were blocked by pretreatment with PTX (50 ng/ml; 16 h) or by the MAPK inhibitor PD98059 (50 μM; ref. 28), suggesting that both G protein and MAPK activation are necessary for the response. This effect is mediated through CREB2, because responses were greatly diminished in the absence of transfected CREB2 (Fig. 6b) and baclofen had no effect on CAT levels from CHO-GABAB cells transfected with a mutated Gadd153 promoter (21) in which the CREB2 recognition site was altered (data not shown).
Figure 6.
CREB2 activates Gadd153 transcription through the GABAB receptor. (a) Dose-response to baclofen. Transfected CHO-GABAB cells were treated with baclofen (0.1 μM-1 mM; 6 h) and cytoplasmic extracts were prepared. CAT levels, from the Gadd153-CAT reporter gene (14), were determined by ELISA assay. Baclofen induced CAT expression in a dose-dependent manner with an EC50 of 4 μM. (b) Effects of CGP54626, PTX, and PD98059 on baclofen-induced CAT production. Cells were pretreated with CGP54626 (1 μM; 30 min), PTX (50 ng/ml; 16 h), or PD98059 (50 μM; 30 min) and CAT levels were determined in cytoplasmic extracts after baclofen treatment (100 μM; 6 h). All three compounds blocked baclofen/CREB2-mediated CAT expression. Transfection of cells with vector (pcDNA3) instead of CREB2 greatly diminished CAT expression levels, and resulted in a markedly decreased response to baclofen.
Discussion
We have shown that the transcription factors CREB2 and ATFx, which share 57% identity over their bZIP domains, both interact with the C-terminal tails of the GABAB receptor by using YTH. This interaction is mediated through the bZIP domains of the two transcription factors and the coiled coil domain in the C terminus of GABAB-R1. We have also detected some interaction with the GABAB-R2 subunit by YTH, although the interaction was through domains other than the bZIP region. The interaction between CREB2 and GABAB-R1 was verified by coimmunoprecipitation of the endogenous proteins from brain extract, but the association did not alter GABAB receptor pharmacology as determined by GTPγS binding. The interaction of the coiled-coil domains of the two GABAB receptor subunits is implicated in receptor dimerization (5, 7), and it is possible that a three-way helical structure exists to connect the GABAB receptor to transcription factors or that some dissociation of the coiled coil occurs within the heterodimer to allow the association with CREB2. We next examined the effect of GABAB receptor activation by baclofen, a specific agonist, on CREB2 localization and function in both recombinant cell systems as well as in rat primary cortical cultures. These data demonstrated that in cells expressing recombinant GABAB receptors or in neurons with endogenous GABAB receptors, CREB2 resides within the cytoplasm rather than the nucleus. More importantly, CREB2 translocated to the nucleus in response to receptor activation, and in a recombinant reporter cell assay, this translocation led to a direct effect on gene transcription. This transcriptional activation involved G protein and MAPK signaling mechanisms, and it occurs possibly thorough G protein βγ (29) activation of the Ras/Raf pathway to promote MAPK activation and phosphorylation of CREB2. Because some CREB2 is already present in the nuclei of neurons, it is doubtful that translocation of CREB2 alone would stimulate gene transcription. Probably, phosphorylation of CREB2 is required for activation of the transcription factor. Certainly, CREB2 is known to possess consensus MAPK phosphorylation sites. This direct interaction between a GPCR and transcription factor represents a previously uncharacterized mode of signaling for the superfamily of 7TM receptors and has some similarity to the Jak/STAT signaling pathway (30). Several GPCRs, including angiotensin AT1 (31), CXCR4 (32), CCR5 (33), 5HT2a (34), and cAR1 (35) couple to the Jak/STAT pathway. Here, ligand binding and receptor dimerization lead to binding of the STAT transcription factors to the receptor, where they are phosphorylated by Jaks. The STATs then dimerize and translocate to the nucleus, where they bind response elements and activate gene expression.
The biological consequences of the GABAB/CREB2 pathway are presently unclear, but significantly, CREB transcription factors have been shown to be central to mechanisms regulating long-term memory and synaptic plasticity (36, 37). This signaling pathway may be important during development of the nervous system. GABAB receptors are highly regulated during development with changes occurring in the levels of the splice variants and subunits (38–40). Changes in the relative levels of GABAB-R1/GABAB-R2 may alter the ability of the GABAB receptor to interact with transcription factors and thereby regulate changes in gene expression during development. Future investigations should determine which genes are regulated through the activation of CREB2 by the GABAB receptor. There are a number of genes that potentially could be activated through the association of CREB2 with the GABAB receptor, and here we show that Gadd153 is a candidate. However, there is no evidence for a role of the GABAB receptor in the stress response as regulated by Gadd153. Interestingly, a number of nerve growth factors, including NGF and BDNF, are known to be regulated by GABA (41) and contain CRE-responsive elements within their promoters. These genes are also potential candidates for regulation by CREB2.
Further studies are now required to determine the exact mechanism by which GABAB receptors alter the cellular localization of CREB2 and how receptor activation leads to translocation of CREB2 to the nucleus to modulate gene expression. The identification of this pathway from synapse to nucleus opens up a new area of research for neuronal receptor signaling and the mechanisms of long-term changes in the nervous system.
Acknowledgments
We thank Nikki Holbrook for supplying the Gadd153 promoter-CAT reporter constructs and Nicola Bevan and Andrew Green for expert technical assistance.
Abbreviations
- GABA
γ-aminobutyric acid
- GPCR
G protein-coupled receptor
- YTH
Yeast two-hybrid
- CHO
Chinese hamster ovary
- eGFP
enhanced green fluorescent protein
- CAT
chloramphenicol acetyltransferase
- MAPK
mitogen-activated protein kinase
- PTX
pertussis toxin
- bZIP
basic leucine zipper
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF305687).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240452197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240452197
References
- 1.Mody I, Koninck Y D, Otis T S, Soltesz I. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 2.Hill D R, Bowery N G. Nature (London) 1981;290:149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- 3.Kaupmann K, Huggel K, Heid J, Flor P J, Bischoff S, Mickel S J, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Nature (London) 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 4.Jones K A, Borowski B, Tamm J A, Craig D A, Durkin M M, Dai M, Yao W J, Johnson M, Gunwaldensen C, Huang L Y, et al. Nature (London) 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 5.White J H, Wise A, Main M, Green A, Fraser N J, Disney G H, Emson P, Foord S, Marshall F H. Nature (London) 1998;396:679–683. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 6.Kaupmann K, Malitshek B, Schuler V, Heid J, Froestl W, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. Nature (London) 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 7.Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau H C. Nature (London) 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 8.Ng G Y, Clark J, Coulombe N, Ethier N, Hebert T E, Sullivan R, Kargman S, Chateauneuf A, Tsukoamoto N, McDonald T, et al. J Biol Chem. 1999;274:7607–7610. doi: 10.1074/jbc.274.12.7607. [DOI] [PubMed] [Google Scholar]
- 9.Couve A, Fillipov A K, Connolly C N, Bettler B, Brown D A, Moss S J. J Biol Chem. 1998;273:26361–26367. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- 10.Kammerer R A, Frank S, Schulthess T, Landwehr R, Lustig A, Engel J. Biochemistry. 1999;38:13263–13269. doi: 10.1021/bi991018t. [DOI] [PubMed] [Google Scholar]
- 11.Leaney J L, Tinker A. Proc Natl Acad Sci USA. 2000;97:5651–5656. doi: 10.1073/pnas.080572297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fillipov A K, Couve A, Pangolos M, Walsh F S, Brown D A, Moss S J. J Neurosci. 2000;20:2867–2874. doi: 10.1523/JNEUROSCI.20-08-02867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sassone-Corsi P. Int J Biochem Cell Biol. 1998;30:27–38. doi: 10.1016/s1357-2725(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 14.Karpinski B A, Morle G D, Huggenvik J, Uhler M D, Leiden J M. Proc Natl Acad Sci USA. 1992;89:4820–4824. doi: 10.1073/pnas.89.11.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang G, Hai T. J Biol Chem. 1997;272:24088–24095. doi: 10.1074/jbc.272.38.24088. [DOI] [PubMed] [Google Scholar]
- 16.Jungling S, Cibelli G, Czardybon M, Gerdes H, Theil G. Eur J Biochem. 1994;226:925–935. doi: 10.1111/j.1432-1033.1994.00925.x. [DOI] [PubMed] [Google Scholar]
- 17.Vallejo M, Ron D, Miller C P, Habener J F. Proc Natl Acad Sci USA. 1993;90:4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishizawa M, Nagata S. FEBS Lett. 1992;299:36–38. doi: 10.1016/0014-5793(92)80094-w. [DOI] [PubMed] [Google Scholar]
- 19.Pati D, Meistrich M L, Plon S E. Mol Cell Biol. 1999;19:5001–5013. doi: 10.1128/mcb.19.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller K J, Morse M A, White J H, Dowell S J, Sims M J. BioTechniques. 1998;25:85–92. doi: 10.2144/98251st04. [DOI] [PubMed] [Google Scholar]
- 21.Fawcett T W, Martindale J L, Guyton K Z, Hai T, Holbrook N J. Biochem J. 1999;339:135–141. [PMC free article] [PubMed] [Google Scholar]
- 22.Osten P, Srivastava S, Inman G J, Vilim F S, Khatri L, Lee L M, States B A, Einheber S, Milner T A, Hanson P I, Ziff E B. Neuron. 1998;21:99–101. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- 23.Weiland T, Jakobs K H. Methods Enzymol. 1994;237:3–13. doi: 10.1016/s0076-6879(94)37048-6. [DOI] [PubMed] [Google Scholar]
- 24.Ciruela F, Soloviev M M, Chan W-Y, McIlhinney R A J. Mol Cell Neurosci. 2000;15:36–50. doi: 10.1006/mcne.1999.0808. [DOI] [PubMed] [Google Scholar]
- 25.Brewer G J, Torricelli J R, Evege E K, Price P J. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber E, Matthias P, Muller M M, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowery N G. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 28.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Ilasaca M. Biochem Pharmacol. 1998;56:269–277. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 30.Cattaneo E, Conti L, De-Fraja C. Trends Neurosci. 1999;22:365–369. doi: 10.1016/s0166-2236(98)01378-2. [DOI] [PubMed] [Google Scholar]
- 31.Marrero M B, Schieffer B, Paxton W G, Heerdt L, Berk B C, Delafontaine P, Bernstein K E. Nature (London) 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 32.Vila-Coro A J, Rodriguez-Frade J M, De Ana A M, Moreno-Ortiz M C, Martinez C, Mellado M. FASEB J. 1999;13:1699–1710. [PubMed] [Google Scholar]
- 33.Rodriguez-Frade J M, Vila-Coro A J, Martin A, Nieto M, Sanchez-Madrid F, Proudfoot A, Wells T, Martinez C, Mellado M. J Cell Biol. 1999;144:755–765. doi: 10.1083/jcb.144.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillet-Deniau I, Burnol A F, Girard J. J Biol Chem. 1997;272:14825–14829. doi: 10.1074/jbc.272.23.14825. [DOI] [PubMed] [Google Scholar]
- 35.Araki T, Gamper M, Early A, Fukuzawa M, Abe T, Kawata T, Kim E, Firtel T, Williams J G. EMBO J. 1998;17:4018–4028. doi: 10.1093/emboj/17.14.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abel T, Kandel E R. Brain Res Rev. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 37.Silva A J, Kogan J H, Frankland P W, Kida S. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 38.Benke D, Honer M, Michel C, Bettler B, Mohler H. J Biol Chem. 1999;274:27323–27330. doi: 10.1074/jbc.274.38.27323. [DOI] [PubMed] [Google Scholar]
- 39.Fritschy J M, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 40.Billinton A, Upton N, Bowery N G. Br J Pharmacol. 1999;126:1387–1392. doi: 10.1038/sj.bjp.0702460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zafra F, Castren E, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]