Abstract
The CD14 receptor is a pattern recognition molecule in the innate immune response against microorganisms and other exogenous and endogenous stress factors. The most important CD14 signalling co-receptor is toll-like receptor 4 (TLR4), which activates, among others, the nuclear factor κB (NF-κB) inflammatory pathway. Besides its role in innate immunity and host defence, the proinflammatory cytokines expressed upon TLR4/NF-κB pathway activation exert proatherogenic effects. The CD14 C(–260)T promoter and TLR4 Asp299Gly functional polymorphisms have been recently implicated in the development of cardiovascular events, suggesting that the genetically determined inflammatory response against pathogens or their antigens may have a major role in atherogenesis and subsequent acute events. Is the association of these polymorphisms with cardiovascular disease more evidence for the implication of infection, especially by Gram negative bacteria, in the development of acute coronary events? This article reviews the molecular basis, biological functions, and clinical implications of the CD14/TLR4 polymorphisms in the development of cardiovascular events.
Keywords: CD14 receptor, toll-like receptor 4, infection, polymorphism, Chlamydia pneumoniae, atherogenesis, acute coronary syndromes
It has been suggested that chronic infection by Gram negative microorganisms may contribute to the inflammatory component of atherosclerosis.1 Endotoxin or lipopolysaccharide (LPS) generated by Gram negative bacteria exerts proatherogenic effects by contributing to low density lipoprotein oxidation, foam cell formation, and thrombogenesis.2 The main receptor for LPS is the CD14 receptor, a pattern recognition molecule of the innate immune system.3 CD14 has been implicated in monocyte activation,4 leucocyte–endothelial cell interactions,5 and regulation of programmed cell death (apoptosis) in both monocytes and endothelial cells,6,7 pivotal processes in the development of atherosclerosis and its complications. The most important CD14 co-receptor, toll-like receptor 4 (TLR4), is responsible for activating intracellular signalling pathways. Functional polymorphisms recently identified in both CD14 promoter and TLR4 genes8,9 have been associated with acute coronary events,10,11 suggesting that the intensity of the genetically determined inflammatory response against pathogens or their antigens may have a major role in determining the magnitude of atherogenesis and subsequent clinical outcome. This article reviews the molecular basis, biological functions, and clinical implications of the CD14/TLR4 polymorphisms in the development of cardiovascular events.
MOLECULAR BASIS
The CD14 receptor is a 356 amino acid membrane glycoprotein12 where the C-terminal leader sequence of 28–30 amino acids is replaced by a glycosyl phosphatidylinositol (GPI) anchor after translation.13 Thus, CD14 is not a transmembrane protein but is anchored to the cellular membrane through GPI linkage. The membrane expressed CD14 (mCD14) is present on the surface of mature myeloid cells14 and differentiation of monocytes into macrophages within different tissues is accompanied by a change in mCD14 receptor number.15,16 GPI anchored proteins, such as mCD14, are clustered in membrane microdomains called “lipid rafts”,17,18 which are implicated in a wide array of cellular processes including transcytosis,19 potocytosis,20 and transmembrane signalling.21 By localising all of the components of specific signalling pathways within a membrane compartment, lipid rafts enable efficient and specific signalling in response to stimuli.17 CD14 also exists as a soluble molecule (sCD14) that can be found with two different molecular weights in serum.22 Various stimuli induce shedding of the GPI anchored mCD14, probably mediated by serine proteases such as leucocyte elastase,23 resulting in sCD14 with a molecular mass of 48–49 kDa. Some CD14 molecules stored intracellularly escape GPI anchor attachment and keep the C-terminal leader sequence, resulting in sCD14 with a molecular weight of 55–56 kDa.22 The sCD14s have an important role in LPS mediated activation of CD14 negative cells (epithelial and smooth muscle cells)16,24 but the biological differences between these two forms are unknown.
Although CD14 receptor per se binds LPS, the presence of serum LPS binding protein (LBP), a 60 kDa acute phase response glycoprotein synthesised mainly in the liver and lung, enhances this interaction 100–1000 times.25 LBP is essential for the rapid induction of an inflammatory response by small amounts of LPS or Gram negative bacteria, as well as for survival upon bacterial infection.26 LBP binds LPS aggregates and catalytically transfers several hundred LPS monomers per LBP molecule, forming a ternary complex with the CD14 receptor.27 The LPS binding domain on CD14 has been located near the amino terminal region of the molecule. Recent single point mutations and monoclonal antibody epitope mapping studies suggest that CD14 can bind multiple microbial ligands in a non-specific fashion by presenting an array of charged residues on one surface of the protein close to each other.28 This tactic is similar to that previously reported for the macrophage scavenger protein.
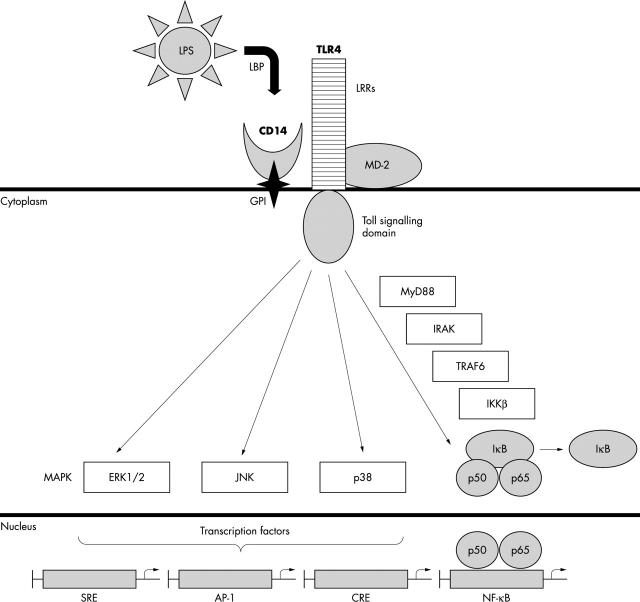
A CD14 co-receptor is needed to activate the intracellular signalling pathways because of the lack of a cytoplasmic domain and the inability of GPI to activate signalling pathways directly.29 The most important of the CD14 co-receptors is TLR4.30 TLRs are members of the interleukin 1 receptor (IL-1R) family,31,32 an evolutionary conserved signalling system that is a critical determinant of the innate immune and inflammatory responses against invading pathogens. The IL-1R family contains several common structural features that include leucine-rich repeats in the ectodomain and a toll/IL-1R cytoplasmic domain. The TLRs share the toll/IL-1R cytoplasmic signalling cascade but are distinguished by their extracellular leucine-rich repeat structure. Two of the human TLRs (TLR4 and TLR2) have been reported to be associated with CD14 receptor and to mediate recognition of cell wall components from Gram negative and Gram positive bacteria, respectively.33 TLR4 activates several intracellular signalling pathways, the most important one being the TLR4/nuclear factor κB (NF-κB) pathway,34 leading to the synthesis and release of antimicrobial peptides, inflammatory cytokines and chemokines, and other costimulatory molecules that provide a link to adaptive immunity (fig 1).35
Figure 1.
CD14/toll-like receptor 4 (TLR4) intracellular signalling complex: lipopolysaccharide (LPS) binds to serum LPS binding protein (LBP) and is transferred to the glycosyl phosphatidylinositol (GPI) linked CD14 receptor, which interacts with the signalling receptor TLR4 and the accessory protein MD-2. Leucine-rich repeats (LRRs) in the extracellular domain of toll family proteins have been implicated in the recognition of pathogens. LPS stimulates the activation of various mitogen activated protein kinase (MAPK) pathways, such as extracellular signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38. These pathways activate various transcription factors, including Elk-1, c-Jun, c-Fos, activating transcription factor 1 (ATF-1), ATF-2, serum response factor, and cAMP response element binding protein. In addition, LPS activates the nuclear factor κB (NF-κB) pathway through myeloid differentiation protein 88 (MyD88), interleukin 1 (IL-1) receptor associated kinase (IRAK), and tumour necrosis factor (TNF) receptor associated factor 6 (TRAF6). Activated inhibitory κB kinase β(IKKβ) phosphorylates inhibitory κB (IκB) and permits nuclear translocation of NF-κB/Rel complexes, such as p50/p65. LPS stimulation of monocytes/macrophages induces many inflammatory mediators, such as cytokines (TNF-α, IL-1, IL-2, IL-6, and granulocyte, macrophage, and granulocyte-macrophage colony stimulation factors), adhesion molecules (E selectin, intercellular adhesion molecule 1, vascular cell adhesion molecule 1), chemokines (IL-8, macrophage chemotactic protein 1), receptors (tissue factor, IL-2Rα), and inflammatory enzymes (inducible nitric oxide synthase and cyclo-oxygenase 2, etc). AP-1, activating protein 1; CRE, cAMP response element; SRE, serum response element.
Although TLR4 alone can activate the intracellular NF-κB signalling pathway, co-expression with MD-2 enhances TLR4 dependent activation of NF-κB and is required to activate or augment the mitogen activated protein kinase pathways and Elk-1 stimulation.36 MD-2, which can also function as a soluble receptor for cells that do not otherwise express it,37 is physically associated with TLR4, and directly influences its specificity. TLR4/MD-2 seems to discriminate LPS from other phospholipids such as those implicated in the recognition of apoptotic bodies.38 Thus, the LPS receptor is a multiprotein complex that consists of at least three proteins, CD14, TLR4, and MD-2 (fig 1).39 Both mCD14 and sCD14, together with LBP, are first line screeners of microbial antigens and present them to the more pathogen specific signalling receptor TLR4-MD-2.16
BIOLOGICAL FUNCTIONS OF CD14/TLR4 AND ATHEROSCLEROSIS
The CD14 receptor is a pattern recognition molecule that has a central role in innate immunity, as it can interact with several ligands, including LPS from Gram negative bacteria and components from Gram positive bacteria and fungi.40–43 Human heat shock protein 60 (hu-HSP60) and hu-HSP70, intracellular proteins thought to be involved in protective functions against cellular stress and infection, and Chlamydia pneumoniae HSP60 (Cp-HSP60) have been reported to activate endothelial cells and monocyte derived macrophages through the CD14 receptor,44–46 although these interactions have been recently questioned.47
The CD14 receptor has been implicated in several biological functions associated with atherosclerosis and its complications. Among these are monocyte activation,5 leucocyte–endothelial cell interactions,6 and regulation of apoptosis.6,7 Monocyte activation by the CD14 receptor induces several intracellular changes that enhance the affinity of monocyte β2 integrins (CD11/CD18) for their ligand, intercellular adhesion molecule 1, on endothelial cells.48–50 This promotes monocyte–endothelium adhesion, which is one of the first and most crucial steps in the development of atherosclerotic lesions. The CD14 receptor has been implicated in the regulation of programmed cell death in both endothelial cells and monocytes. LPS has been shown to trigger apoptosis in endothelial cells through an sCD14 dependent mechanism.7 On the other hand, LPS induced increase in CD14 expression promotes survival of monocytes, whereas downregulated CD14 expression evokes apoptosis.7 Protection against apoptosis induced by CD14 dependent NF-κB activation is due to an induction of antiapoptotic factors, such as mitochondrial antiapoptotic factor Bcl-2,51 inhibitor of apoptosis protein 1,52 or X linked inhibitor of apoptosis protein,53 which inhibit several of the caspase enzymes involved in the cell death programme. Resistance of macrophages to apoptotic triggers may be beneficial for inflammatory processes where macrophages are needed as phagocytes for removal of moribund cells and apoptotic bodies, processes in which the CD14 receptor has also been implicated.54 Apoptotic cells are recognised by different cellular systems, such as the phosphatidylserine receptor, the CD36/αvβ3 integrin/thrombospondin system, and the lectin and CD14 receptor systems.55,56 However, apoptotic cells, unlike LPS, do not provoke the release of proinflammatory cytokines from macrophages.55 Therefore, the multifunctional CD14 receptor is a surface molecule of monocytes that can promote survival and antagonise apoptosis and a recognition receptor of macrophages that enables interaction with apoptotic cells.57
CD14 PROMOTER POLYMORPHISM AND ATHEROMATOUS PLAQUE INSTABILITY
The CD14 receptor is considered to be a monocyte activation marker,58,59 and both increased density of mCD14 and serum concentrations of sCD1458,59 have been reported in patients with acute coronary syndromes (ACS). However, it has been shown that the CD14 receptor is not merely a monocyte activation marker, as it can synergise with C reactive protein in the activation of endothelium,60 considered to be the first step in atherogenesis and coronary events. Moreover, increased monocytic CD14 expression during ACS was associated with a 2.4-fold higher secretion of tumour necrosis factor α by infectious stimuli (LPS).59 Thus, patients with increased monocyte CD14 expression may have an enhanced inflammatory response to LPS or other Gram negative bacteria products (that is, Cp-HSP60) and this may contribute to the development of ACS.
A recently identified single nucleotide polymorphism (C→T) in position −260 of the CD14 promoter has been shown to increase transcriptional activity by lowering the affinity of the GC box for Sp3,8 a factor known to inhibit the activity of a number of promoters. This enhanced transcriptional activity has been associated with higher concentrations of sCD14,61 enhanced expression of mCD1462 on monocytes, and with the risk of myocardial infarction (MI)(table 1).10,62–70
Table 1.
Results from studies investigating the potential association between risk of myocardial infarction and the C(−260)T polymorphism in the promoter of the CD14 receptor gene
| Author and reference | n | Cases | Controls | Association | Comments | ||
| Allele T | Genotype T/T | Allele T | Genotype T/T | ||||
| Unkelbach et al10 | 2228* | 48% | 23% | 46% | 21% | No | Association in low risk patients, especially >62 years |
| Hubacek et al62 | 313* | 49% | 28% | 35% | 16% | Yes | OR 1.8, 95% CI 1.3 to 2.5, p<0.05† |
| Shimada et al63 | 164* | 65% | 52% | 49% | 23% | Yes | OR 3.8, 95% CI 1.5 to 9.4, p<0.05)† |
| Zee et al68 | 774‡ | 47% | 19% | 47% | 22% | No | Association in >60 years and BMI >25 kg/m2 |
| Nauck et al69 | 2062* | 48% | 24% | 47% | 21% | No | None |
| Koch et al67 | 1895* | 46% | 22% | 48% | 22% | No | None |
| Hohda et al64 | 1029* | 57% | 33% | 52% | 25% | Yes | OR 1.4, 95% CI 1.1 to 1.9, p<0.05† |
| Arroyo-Espliguero et al65 | 428* | 55% | 33% | 45% | 22% | Yes | OR 3.1, 95% CI 1.3 to 7.4, p<0.05† |
| Longobardo et al70 | 430* | 56% | 33% | 51% | 27% | No | None |
| Kondo et al66 | 333* | NA | NA | NA | NA | No | Association in patients with myocardial infarction with insignificant coronary artery stenosis (OR 3.32, 95% CI 1.38 to 8.12) |
*Case–control studies;†odds ratios of genotype T/T;‡nested case–control study.
BMI, body mass index; CI, confidence interval; NA, not available; OR, odds ratio.
Unkelbach et al10 found an increased risk of MI among homozygous carriers of the T allele with a low atherosclerotic risk profile. Three other studies involving Czech62 and Japanese63,64 populations showed an increased risk of MI associated with the T allele and the T/T genotype of the CD14 promoter polymorphism. We have found that the T/T genotype was also associated with a higher prevalence of ACS among patients with coronary artery disease.65 Thus, the CD14 receptor seems to have a role in atheromatous plaque vulnerability and may be considered a genetically determined risk factor for ACS. In fact, it has been recently reported that the T/T genotype was prevalent among patients with MI with insignificant coronary artery stenosis at the culprit lesion and that peripheral blood mononuclear cells from T/T homozygotes release a large amount of tumour necrosis factor α when challenged with LPS, more than C/T or C/C genotypes.66 Taking these data together and given that the density of the CD14 receptor has been reported to be higher in T/T homozygotes than in the other two genotypes,62 coronary plaques of patients carrying the T/T genotype have a tendency to rupture because of the existence of activation prone monocytes/macrophages, even when there is only a small amount of coronary atheroma.66 This supports the role of CD14 promoter polymorphism in atheromatous plaque vulnerability. However, findings of an association between the T allele of the CD14 promoter polymorphism and the risk of MI are not universal (table 1). Recent studies found no association between allele and genotype distributions of CD14 promoter polymorphism and the risk of MI.67–70 The discrepancies between these negative studies and those where an association has been found may be explained by a different ethnic background of the study population and the use of subgroup analysis. Moreover, most of the CD14 studies were cross sectional and not prospective studies. Therefore, the reported association between CD14 promoter polymorphism and the risk of MI may reflect a real susceptibility of patients who carry the T allele but it may also reflect either linkage disequilibrium or be a chance finding. Thus, prospective studies are needed to further clarify this issue.
Despite the discrepancies, the weight of the evidence points to a higher vascular risk among patients with proinflammatory gene polymorphisms,71 and a potential role of the CD14 promoter polymorphism in the risk of ACS can be proposed. Chronic infection and recent reinfection by C pneumoniae have been associated with the development of acute coronary events.72 Chlamydial LPS antigen and Cp-HSP60 released from damaged tissues of distant sites of infection or inflammation may trigger proinflammatory responses through the CD14 receptor in atheromatous plaque infiltrating monocytes/macrophages. The signalling receptor for LPS and for both Cp-HSP60 and hu-HSP60 is the CD14 co-receptor TLR4.30 Recently, it has been shown that TLR4 is preferentially expressed by macrophages within lipid rich atherosclerotic lesions and is upregulated by oxidised low density lipoprotein.73 Normal arteries have only minimal or no TLR4 expression. Thus, the enhanced expression of CD14 and TLR4 on monocytes/macrophages, associated with the C(−260)T polymorphism and by oxidised low density lipoprotein of lipid rich atherosclerotic plaque, respectively, may fuel the inflammatory response of monocytes to infection by C pneumoniae or its components and therefore promote plaque vulnerability in patients with coronary artery disease. The exaggerated immune response to C pneumoniae or its products, as reflected by the increased monocyte responsiveness to LPS stimulation in patients with recurrent unstable angina,74 may be mediated by these two mechanisms and implicated in atheromatous plaque instability. Moreover, seropositivity for Cp-HSP60 appears to be a sensitive and specific marker of ACS, unrelated to C pneumoniae IgG antibody titres or C reactive protein concentrations.75 These findings suggest an enhanced anti-self immune response related to antigenic mimicry between Cp-HSP60 and hu-HSP60 (and not directly related to C pneumoniae infection), possibly also CD14/TLR4 mediated.
TLR4 POLYMORPHISM AND CARDIOVASCULAR EVENTS
A few genetic variants of TLR4 that affect composition, structure, and function of the receptor have been identified.9 The most frequent polymorphisms, TLR4 Asp299Gly and Thr399Ile, both affect the extracellular domain of the receptor.9 The Asp299Gly variant is biologically important, as it attenuates TLR4 mediated LPS signalling and is associated with a diminished airway response to inhaled LPS in healthy volunteers.9 Recent results from the prospective population based Bruneck study showed that patients with the Asp299Gly TLR4 polymorphism had a significantly lower risk of early plaque development in the carotid arteries.76 This study also showed that the cumulative burden of cardiovascular disease was reduced by more than half in patients with both 399Ile and 299Gly TLR4 polymorphisms, as compared with the background population. Moreover, a decreased risk of acute coronary events has also been reported among carriers of the 299Gly TLR4 polymorphism.11 Further indirect support for a protective role of the Asp299Gly TLR4 polymorphism in human atherogenesis derives from the association of this genetic variant with low concentrations of C reactive protein, adhesion molecules, and other acute phase reactant and inflammatory molecules.76
These data, and those for the CD14 promoter polymorphism, support the concept that the intensity of the genetically determined individual inflammatory response may have a major role in determining the magnitude of atherogenesis and subsequent clinical outcome. As underlined in a recent review,77 the selection of genes with an enhanced inflammatory response against infection from earlier times may have switched to a maladaptive response in our modern environment. This genetic variance in the innate immune system may be associated with clinical events related to atheromatous plaque rupture. Taking all this information together, it is tempting to speculate that a CD14/TLR4 mediated link exists between infection and the development of acute coronary events.
CONCLUSION
CD14 and the TLR4/NF-κB pathway have been associated with various biological functions implicated in the development of atherosclerosis and acute coronary events. The C(−260)T polymorphism in the promoter of the CD14 receptor gene is associated with enhanced transcriptional activity,8 increased CD14 expression,61,62 and acute coronary events.10,62,63,66 The 299Gly TLR4 polymorphism, which is associated with attenuated receptor signalling, is related to the risk of developing acute severe infections but has been associated with low concentrations of circulating mediators of inflammation and a decreased risk of atherogenesis and ACS.11,76 The presence of gene polymorphisms with proinflammatory associations may fuel the inflammatory response of monocytes/macrophages to infection by C pneumoniae or its components and therefore promote atheromatous plaque vulnerability. Further well designed prospective trials are needed to understand fully the role of genetic polymorphisms in atherogenesis and the development of cardiovascular events.
Abbreviations
ACS, acute coronary syndromes
Cp-HSP, Chlamydia pneumoniae heat shock protein
GPI, glycosyl phosphatidylinositol
hu-HSP, human heat shock protein
IL-1R, interleukin 1 receptor
LBP, lipopolysaccharide binding protein
LPS, lipopolysaccharide
mCD14, membrane expressed CD14
MI, myocardial infarction
NF-κB, nuclear factor κB
sCD14, soluble CD14
TLR4, toll-like receptor 4
REFERENCES
- 1.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation 1999;100:e20–8. [DOI] [PubMed] [Google Scholar]
- 2.Liao W . Endotoxin: possible roles in initiation and development of atherosclerosis. J Lab Clin Med 1996;128:452–60. [DOI] [PubMed] [Google Scholar]
- 3.Wight SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431–3. [DOI] [PubMed] [Google Scholar]
- 4.Ulevitch RJ, Tobias PS. Recognition of Gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol 1999;11:19–22. [DOI] [PubMed] [Google Scholar]
- 5.Beekhuizen H , Blokland I, Corsel-van Tilburg A, et al. CD14 contributes to the adherence of human monocytes to cytokine-stimulated endothelial cells. J Immunol 1991;147:3761–7. [PubMed] [Google Scholar]
- 6.Heidenreich S , Schmidt M, August C, et al. Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol 1997;159:3178–88. [PubMed] [Google Scholar]
- 7.Frey EA, Finlay BB. Lipopolysaccharide induces apoptosis in a bovine endothelial cell line via a soluble CD14 dependent pathway. Microb Pathog 1998;24:101–9. [DOI] [PubMed] [Google Scholar]
- 8.LeVan TD, Bloom JW, Bailey TJ, et al. A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol 2001;167:5838–44. [DOI] [PubMed] [Google Scholar]
- 9.Arbour NC, Lorenz E, Schutte BC. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 2000;25:187–91. [DOI] [PubMed] [Google Scholar]
- 10.Unkelbach K , Gardemann A, Kostrzewa M, et al. A new promoter polymorphism in the gene of lipopolysaccharide receptor CD14 is associated with expired myocardial infarction in patients with low atherosclerotic risk profile. Arterioscelr Thromb Vasc Biol 1999;19:932–8. [DOI] [PubMed] [Google Scholar]
- 11.Ameziane N , Beillat T, Verpillat P, et al. Association of the toll-like receptor 4 Asp299Gly polymorphism with acute coronary syndromes. Arterioscler Thromb Vasc Biol 2003;23:e61–4. [DOI] [PubMed] [Google Scholar]
- 12.Ferrero E , Goyert SM. Nucleotide sequence of the gene encoding the monocyte differentiation antigen, CD14. Nucl Acids Res 1988;16:4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haziot A , Chen S, Ferrero E, et al. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol 1988;141:547–52. [PubMed] [Google Scholar]
- 14.Goyert SM, Ferrero EM, Seremetis SV, et al. Biochemistry and expression of myelomonocytic antigens. J Immunol 1986;137:3909–14. [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock HW, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today 1993;14:121–5. [DOI] [PubMed] [Google Scholar]
- 16.Antal-Szalmás P . Evaluation of CD14 in host defense. Eur J Clin Invest 2000;30:167–79. [DOI] [PubMed] [Google Scholar]
- 17.Zajchowski LD, Robbins SM. Lipid rafts and little caves: compartmentalized signalling in membrane microdomains. Eur J Biochem 2002;269:737–52. [DOI] [PubMed] [Google Scholar]
- 18.Simons K , Toomre D. Lipid rafts and signal transduction. Nat Rev 2000;1:31–41. [DOI] [PubMed] [Google Scholar]
- 19.Simionescu N . Cellular aspects of transcapillary exchange. Physiol Rev 1983;63:1536–79. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RG, Kamen BA, Rothberg KG, et al. Potocytosis: sequestration and transport of small molecules by caveolea. Science 1992;255:410–1. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T , Schlegel A, Scherer PE, et al. Caveolins, a family of scaffolding proteins for organizing ‘pre-assembled signaling complexes’ at the plasma membranes. J Biol Chem 1998;273:5419–22. [DOI] [PubMed] [Google Scholar]
- 22.Labeta MO, Durieux JJ, Fernández N, et al. Release from a human monocyte-like cell line of two different soluble forms of the lipopolysaccharide receptor, CD14. Eur J Immunol 1993;23:2144–51. [DOI] [PubMed] [Google Scholar]
- 23.Le-Barillec K , Si-Tahar M, Balloy V, et al. Proteolysis of monocyte CD14 by human leukocyte elastase inhibits lipopolysaccharide-mediated cell activation. J Clin Invest 1999;103:1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loppnow H , Stelter F, Schonbeck U, et al. Endotoxin activates human vascular smooth muscle cells despite lack of expression of CD14 mRNA or endogenous membrane CD14. Infect Immun 1995;63:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hailman E , Lichenstein HS, Wurfel MM, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med 1994;179:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamping N , Dettmer R, Schroder NW, et al. LPS-binding protein protects mice from septic shock caused by LPS of gram-negative bacteria. J Clin Invest 1998;101:2065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobias PS, Soldau K, Gegner JA, et al. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem 1995;270:10482–8. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham MD, Shapiro RA, Seachord C, et al. CD14 employs hydrophilic regions to “capture” lipopolysaccharides. J Immunol 2000;164:3255–63. [DOI] [PubMed] [Google Scholar]
- 29.Lee JD, Kravchenko V, Kirkland TN, et al. Glycosyl-phosphatidylinositol-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc Natl Acad Sci USA 1993;90:9930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poltorak A , He X, Smirnova I, et al. Defective LPS signalling in C3H/HeJ and C57BL/10ScCR mice: mutation in TLR4 gene. Science 1998;282:2085–8. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov R , Preston-Hurlburt P, Janeway CA Jr. A human homologue of the drosophila toll protein signals activation of adaptive immunity. Nature 1997;388:394–7. [DOI] [PubMed] [Google Scholar]
- 32.O’Neill LA. The interleukin-1 receptor/toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2000;2000:RE1. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi O , Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacteria cell wall components. Immunity 1999;11:443–51. [DOI] [PubMed] [Google Scholar]
- 34.Guha M , Mackman N. LPS induction of gene expression in human monocytes. Cell Signal 2001;13:85–94. [DOI] [PubMed] [Google Scholar]
- 35.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 1997;336:1066–71. [DOI] [PubMed] [Google Scholar]
- 36.Yang H , Young DW, Gusovsky F, et al. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem 2000;275:20861–6. [DOI] [PubMed] [Google Scholar]
- 37.Visintin A , Mazzoni A, Spitzer JA, et al. Secreted MD-2 is a large polymeric protein that efficiently confers lipopolysaccharide sensitivity to toll-like receptor 4. Proc Natl Acad Sci USA 2001;98:12156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akashi S , Ogata H, Kirikae T, et al. Regulatory roles for CD14 and phosphatidylinositol in the signaling via toll-like receptor 4-MD-2. Biochem Biophys Res Commun 2000;268:172–7. [DOI] [PubMed] [Google Scholar]
- 39.da Silva J , Ulevitch RJ. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J Biol Chem 2002;277:1845–54. [DOI] [PubMed] [Google Scholar]
- 40.Savedra R Jr, Delude RL, Ingalls RR, et al. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the endotoxin signaling system. J Immunol 1996;157:2549–54. [PubMed] [Google Scholar]
- 41.Sellati TJ, Bouis DA, Kitchens RL, et al.Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol 1998;160:5455–64. [PubMed] [Google Scholar]
- 42.Dziarski R , Tapping RI, Tobias PS. Binding of bacterial peptidoglycan to CD14. J Biol Chem 1998;273:8680–90. [DOI] [PubMed] [Google Scholar]
- 43.Klein BS. Role of cell surface molecules of Blastomyces dermatidis in the pathogenesis and immunobiology of blastomycosis. Semin Respir Infect 1997;12:198–205. [PubMed] [Google Scholar]
- 44.Kol A , Lichtman AH, Finberg RW, et al. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol 2000;164:13–7. [DOI] [PubMed] [Google Scholar]
- 45.Bulut Y , Faure E, Thomas L, et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol 2002;168:1435–40. [DOI] [PubMed] [Google Scholar]
- 46.Dybdahl B , Wahba A, Lien E, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signalling through toll-like receptor-4. Circulation 2002;105:685–90. [DOI] [PubMed] [Google Scholar]
- 47.Gao B , Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor α from murine macrophages. J Biol Chem 2003;278:22523–9. [DOI] [PubMed] [Google Scholar]
- 48.Lauener RD, Geha RS, Vercelli D. Engagement of the monocyte surface antigen CD14 induces lymphocyte function-associated antigen-1/intercellular adhesion molecule-1-dependent homotypic adhesion. J Immunol 1990;145:1390–4. [PubMed] [Google Scholar]
- 49.Wright SD, Ramos RA, Hermanowski-Vosatka S, et al. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med 1991;173:1281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beekhuizen H , Blokland I, van Furth R. Cross-linking of CD14 molecules on monocytes results in a CD11/CD18- and ICAM-1-dependent adherence to cytokine-stimulated human endothelial cells. J Immunol 1993;150:950–9. [PubMed] [Google Scholar]
- 51.Maulik N , Goswami S, Galang N, et al. Differential regulation of Bcl-2, AP-1, and NF-κB on cardiomyocyte apoptosis during myocardial ischemic stress adaptation. FEBS Lett 1999;443:331–6. [DOI] [PubMed] [Google Scholar]
- 52.Liston P , Roy N, Tamai K, et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 1996;379:349–53. [DOI] [PubMed] [Google Scholar]
- 53.Hida A , Kawakami A, Nakashima T, et al. Nuclear factor-kappaB and caspases co-operatively regulate the activation and apoptosis of human macrophages. Immunology 2000;99:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heidenreich S . Monocyte CD14: multifunctional receptor engaged in apoptosis from both sides. J Leukoc Biol 1999;65:737–43. [DOI] [PubMed] [Google Scholar]
- 55.Messmer UK, Pfeilschifter J. New insights into the mechanism for clearance of apoptotic cells. BioEssays 2000;22:878–81. [DOI] [PubMed] [Google Scholar]
- 56.Devitt A , Moffatt OD, Raykundalia C, et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 1998;392:505–9. [DOI] [PubMed] [Google Scholar]
- 57.Heidenreich S . Monocyte CD14: a multifunctional receptor engaged in apoptosis from both sides. J Leukoc Biol 1999;65:737–43. [DOI] [PubMed] [Google Scholar]
- 58.Zalai CV, Koloziejczyk MD, Pilarski L, et al. Increased circulating monocyte activation in patients with unstable coronary syndromes. J Am Coll Cardiol 2001;38:1340–7. [DOI] [PubMed] [Google Scholar]
- 59.Lee WH, Lee Y, Jeong JO, et al. Activation of CD14 on circulating monocytes in patients with acute coronary syndromes. Int J Cardiol 2001;80:135–42. [DOI] [PubMed] [Google Scholar]
- 60.Palusinski RP, Vaisman D, Pasceri V, et al. Synergy between CD14 and C-reactive protein (CRP) in endothelial cell activation. Circulation 2002;106 (suppl II) :533–4. [Google Scholar]
- 61.Baldini M , Lohman IC, Halonen M, et al. A polymorphism in the 5′ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999;20:976–83. [DOI] [PubMed] [Google Scholar]
- 62.Hubacek JA, Pit’ha J, Škodová Z, et al. C(-260)→T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation 1999;99:3218–20. [DOI] [PubMed] [Google Scholar]
- 63.Shimada K , Watanabe Y, Mokuno H, et al. Common polymorphism in the promoter of the CD14 monocyte receptor gene is associated with acute myocardial infarction in Japanese men. Am J Cardiol 2000;86:682–4. [DOI] [PubMed] [Google Scholar]
- 64.Hohda S , Kimura A, Sasaoka T, et al. Association study of CD14 polymorphism with myocardial infarction in a Japanese population. Jpn Heart J 2003;44:613–22. [DOI] [PubMed] [Google Scholar]
- 65.Arroyo-Espliguero R , El-Sharnouby K, Vázquez-Rey E, et al. CD14 C(−260)T polymorphism and prevalence of acute coronary syndromes. Int J Cardiol 2004; (in press). [DOI] [PubMed]
- 66.Kondo T , Ohno M, Shimokata K, et al. CD14 promoter polymorphism is associated with acute myocardial infarction resulting from insignificant coronary artery stenosis. Heart 2003;89:931–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koch W , Kastrati A, Mehilli J, et al. CD14 gene–159C/T polymorphism is not associated with coronary artery disease and myocardial infarction. Am Heart J 2002;143:971–6. [DOI] [PubMed] [Google Scholar]
- 68.Zee RYL, Lindpaintner K, Struk B, et al. A prospective evaluation of the CD14 C(−260)T gene polymorphism and the risk of myocardial infarction. Atherosclerosis 2001;154:699–702. [DOI] [PubMed] [Google Scholar]
- 69.Nauck M , Winkelmann BR, Hoffmann MM, et al. C(−260)T polymorphism in the promoter of the CD14 gene is not associated with coronary artery disease and myocardial infarction in the Ludwigshafen risk and cardiovascular health (LURIC) study. Am J Cardiol 2002;90:1249–52. [DOI] [PubMed] [Google Scholar]
- 70.Longobardo MT, Cefalú AB, Pezzino F, et al. The C(−260)T gene polymorphism in the promoter of the CD14 monocyte receptor gene is not associated with acute myocardial infarction. Clin Exp Med 2003;3:161–5. [DOI] [PubMed] [Google Scholar]
- 71.Andreotti F , Porto I, Crea F, et al. Inflammatory gene polymorphism and ischaemic heart disease: review of population association studies. Heart 2002;87:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandra HR, Choudhary N, O’Neill C, et al.Chlamydia pneumoniae exposure and inflammatory markers in acute coronary syndromes (CIMACS). Am J Cardiol 2001;88:214–8. [DOI] [PubMed] [Google Scholar]
- 73.Xu XH, Shah PK, Faure E, et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerosis plaques and upregulated by oxidized LDL. Circulation 2001;104:3103–8. [DOI] [PubMed] [Google Scholar]
- 74.Liuzzo G , Angiolillo DJ, Buffon A, et al. Enhanced response of blood monocytes to in vitro lipopolysaccharide-challenge in patients with recurrent unstable angina. Circulation 2001;103:2236–41. [DOI] [PubMed] [Google Scholar]
- 75.Biasucci LM, Liuzzo G, Ciervo A, et al. Antibody response to chlamydia heat shock protein 60 is strongly associated with acute coronary syndromes. Circulation 2003;107:3015–7. [DOI] [PubMed] [Google Scholar]
- 76.Kiechl S , Lorenz E, Reindl M, et al. Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med 2002;347:185–92. [DOI] [PubMed] [Google Scholar]
- 77.Kiechl S , Wiedermann CJ, Willeit J. Toll-like receptor 4 and atherogenesis. Ann Med 2003;35:164–71. [DOI] [PubMed] [Google Scholar]