It is assumed that the athlete’s heart represents a physiological adaptation to endurance exercise, caused by an increased volume load.1 As a new cardiac biomarker, brain natriuretic peptide (BNP) allows us to serologically perceive myocardial wall stress. As elevated resting BNP concentrations reflect pathological ventricular overload, it was hypothesised that in case of a physiological hypertrophy BNP concentrations have to be similar in endurance athletes with athlete’s heart and healthy untrained control subjects.
In contrast to atrial natriuretic peptide, which refers to atrial stretch, BNP refers to ventricular strain. Synthesised by cardiomyocytes in response to ventricular overload and elevated myocardial wall stress,2 resting concentrations of BNP and its cleaved precursor peptide N-terminal pro-BNP (NT-proBNP) have become useful markers to assess cardiac dysfunction, chronic heart failure, and left ventricular (LV) hypertrophy caused by hypertension or cardiomyopathy. In British Army recruits an association between the increase in LV mass and elevated resting BNP concentrations after a 10 week training period was demonstrated by Montgomery and colleagues3 However, in another study, BNP concentrations did not correlate with echocardiographically determined LV mass in a small group of eight cyclists with athlete’s heart.4
Therefore, we determined NT-proBNP in a subgroup of previously reported healthy male endurance athletes and healthy untrained control subjects, which were examined by cardiac magnetic resonance (CMR) imaging to determine LV and right ventricular (RV) mass, end diastolic volume (EDS), end systolic volume (ESV), and ejection fraction (EF).5 To confirm the assumption that the athlete’s heart represents a physiological adaptation to endurance exercise, it was hypothesised that resting NT-proBNP concentrations of endurance athletes are similar to healthy untrained subjects with hearts of normal size.
METHODS
The study was approved by the institutional review committee and all participants gave their written informed consent. Twenty well trained healthy male endurance athletes of regional up to international top class (group A), including 10 triathletes, five road cyclists, and five long distance runners (age, 28 (4) years; height, 178(7) cm; weight, 69 (8) kg; heart volume, 14.5 (1.1) ml/kg; aerobic capacity Vo2max, 68 (6) ml/min/kg; endurance training volume per week, 17 (6) hours; training history, 8 (4) years) and a control group of 20 healthy untrained males (group C) (age, 26 (4) years; height, 179 (6) cm; weight, 73 (8) kg; heart volume, 10.9 (0.6) ml/kg; Vo2max, 42 (5) ml/min/kg) matched for age, weight and height with no history of physical activity were included into the study. Vo2max, CMR, and echocardiographic parameters were determined as described previously.5 Diastolic function was determined by pulsed Doppler spectral recordings in the four chamber view at the tips of the mitral leaflets and an E/A ratio > 1 was considered to be normal. In addition to isolated CMR determinations of LV and RV cardiac output (LV-CO, RV-CO) and cardiac index (LV-CI, RV-CI),5 the mean cardiac output (mean CO) and the mean cardiac index (mean CI) were averaged for each subject: mean CO = (LV-CO + RV-CO)/2; mean CI = (LV-CI + RV-CI)/2.
NT-proBNP was measured by an electrochemiluminscence assay using the automated assay of Roche Diagnostics (Elecsys proBNP). The sensitivity of the test is 5 pg/ml. Intra- and interassay coefficients of variance at 175 pg/ml are 2.7% and 3.2%, respectively. There are no cross reactions with other hormones or pharmaceutical drugs.
Normality was tested by the Kolmogorov-Smirnov test and differences between endurance athletes and control subjects by Student’s t test for independent samples. As NT-proBNP was not normally distributed, the Mann-Whitney test was used for the comparison between endurance athletes and untrained subjects. Spearman’s rank coefficient was used to test correlations between NT-proBNP and selected variables. Data are expressed as mean (SD), except NT-proBNP data (median and centiles). Differences were considered to be significant with an α error level of p < 0.05. To accept the hypothesis of similar NT-proBNP values between endurance athletes and control subjects the p value had to be > 0.20.
RESULTS
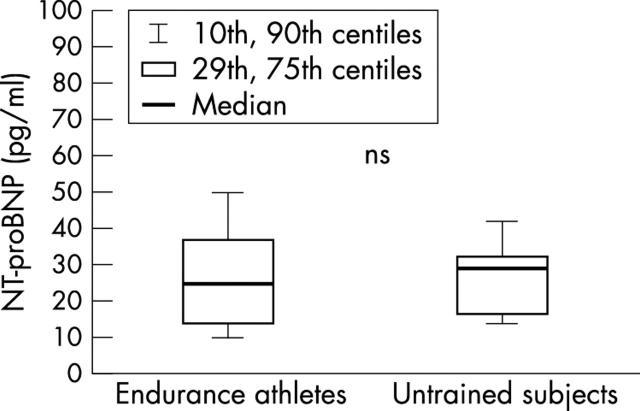
No difference was found in diastolic or systolic function between groups (E/A ratio for group A, endurance athletes, 1.7 (0.3) and group C, untrained control subjects, 1.6 (0.3); echocardiographic fractional shortening: group A, 36 (5)% v group C, 37 (4)%; magnetic resonance imaging derived LV ejection fraction: group A, 59 (3)% v group C, 59 (6)%). There was no difference in NT-proBNP values between endurance athletes and untrained control subjects (p = 0.56); endurance athletes, 24.7 pg/ml (10th, 25th, 75th, and 90th centiles: 9.9, 14.1, 37.3, and 49.4 pg/ml, respectively) and untrained control subjects, 28.9 pg/ml (10th, 25th, 75th and 90th centiles: 14.1, 16.7, 32.5, and 41.2 pg/ml, respectively) (fig 1).
Figure 1.
NT-proBNP concentrations at rest in endurance athletes with athlete’s heart and in healthy untrained control subjects. Box and whisker plots show medians and 10th, 25th, 75th, and 90th centiles.
Resting mean CO (5.2 (0.9) v 5.1 (0.9) l/min) and mean CI (2.8 (0.4) v 2.7 (0.6) l/min/m2) were similar in endurance athletes and untrained control subjects. Moderate but significant correlations (p < 0.05) were found for the relation between NT-proBNP and mean CO (r = 0.67), NT-proBNP and mean CI (r = 0.52), NT-proBNP and LV-CO (r = 0.56), and NT-proBNP and RV-CO (r = 0.59). All other parameters revealed only weak or insignificant correlations with NT-proBNP (NT-proBNP and LV-CI, r = 0.39; NT-proBNP and RV-CI, r = 0.47; NT-proBNP and RV-EDV, r = 0.39; NT-proBNP and RV-SV, r = 0.36).
DISCUSSION
In the present study, NT-proBNP concentrations did not differ between male endurance athletes with athlete’s heart and healthy untrained control subjects with hearts of normal size. As NT-proBNP represents BNP in an equimolar manner, the present results support the observation of Almeida and colleagues, who also found no difference between BNP concentrations in a small group of eight cyclists and their age matched controls.4 Although Almeida and colleagues reported an unrealistically high LV mass index determined by echocardiography, we also did not find a relation between LV mass and resting NT-proBNP concentration even when examining a greater number of subjects using the in vivo gold standard CMR.
Therefore, the present results do not support the findings of Montgomery and colleagues, who observed an increase in BNP concentrations in angiotensin converting enzyme D-allele-positive healthy male British Army recruits with an increase in LV mass after 10 weeks of intensive strength and endurance training.3 As a marker of myocardial growth,3 the raised BNP concentrations reported by Montgomery might reflect acute cardiac stress and a beginning myocardial adaptation to the training stimulus (acute effect), whereas in endurance athletes with a longer history of training, the myocardium might be already adapted to endurance exercise bouts without further induction of relevant cardiomyocyte growth. Consequently, BNP concentrations do not remain elevated in endurance athletes with athlete’s heart at rest (chronic effect).
Furthermore, in the present study the NT-proBNP concentrations of both groups were similar to those values reported for normal healthy individuals and did not exceed the upper limit of 100 pg/ml.2
In conclusion, as a marker of myocardial wall stress, the described similarity of NT-proBNP values between male endurance athletes with athlete’s heart and healthy untrained control subjects with hearts of normal size supports the hypothesis that repeated bouts of endurance exercise do not chronically alter myocardial integrity and that myocardial wall stress is not elevated in endurance athletes with athlete’s heart. The results confirm the assumption that the athlete’s heart represents a physiological hypertrophy to an increased volume load without ventricular overload. Therefore, under resting conditions, NT-proBNP (and BNP) might be a useful additional tool to differentiate between physiological and pathological cardiac hypertrophy.
Abbreviations
BNP, brain natriuretic peptide
CI, cardiac index
CMR, cardiac magnetic resonance
CO, cardiac output
EF, ejection fraction
EDV, end diastolic volume, ESV, end systolic volume
LV, left ventricular
NT-proBNP, N-terminal pro-brain natriuretic peptide
RV, right ventricular
REFERENCES
- 1.Urhausen A , Kindermann W. Sports-specific adaptations and differentiation of the athlete’s heart. Sports Med 1999;28:237–44. [DOI] [PubMed] [Google Scholar]
- 2.Maisel A . B-type natriuretic peptide levels: a potential novel “white count” for congestive heart failure. J Card Fail 2001;7:183–93. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery H , Clarkson P, Dollery C, et al. Association of angiotensin-converting enzyme gene I/D polymorphism with change in left ventricular mass in response to physical training. Circulation 1997;96:741–7. [DOI] [PubMed] [Google Scholar]
- 4.Almeida S , Azevedo A, Castro A, et al. B-type natriuretic peptide is related to left ventricular mass in hypertensive patients but not in athletes. Cardiology 2002;98:113–5. [DOI] [PubMed] [Google Scholar]
- 5.Scharhag J , Schneider G, Urhausen A, et al. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained control subjects determined by magnetic resonance imaging. J Am Coll Cardiol 2002;40:1856–63. [DOI] [PubMed] [Google Scholar]