Abstract
A family of octapeptide derivatives of somatostatin cyclized via a disulfide bridge (des-AA1,2,4,5,12,13[d-2Nal8]-somatostatin-14, ODN-8) was identified that has high affinity and selectivity for the human sst3 somatostatin receptor subtype transfected in CCL39 cells. The binding affinity of carbamoyl-des-AA1,2,4,5,12,13[d-Cys3,Tyr7,d-Agl8(Me,2-naphthoyl)]-somatostatin-14 (sst3-ODN-8) is equal to that of somatostatin-28 for sst3 and less than one-thousandth that for the other four somatostatin receptor subtypes. Compound sst3-ODN-8 potently reverses the somatostatin-28-induced inhibition of forskolin-stimulated cAMP production (pKB = 9.07) and reverses the somatostatin-28-induced stimulation of phospholipase C activity (pKi = 9.22) in sst3-transfected CCL39 cells. [125I-Tyr7]sst3-ODN-8 selectively labels sst3-expressing cells with subnanomolar binding affinity (KD = 0.27 nM). With the use of this radioligand, sst3-expressing human tumors, particularly inactive pituitary adenomas, can be identified with receptor autoradiography; moreover, areas of the human lymphoreticular system express sst3 binding sites selectively displaced by nanomolar concentrations of sst3-ODN-8. Based on the structure–activity relationship of selected analogs substituted at positions 3, 7, and 8, we hypothesize that the basis for sst3 selectivity, high affinity, and possibly antagonism resides in the ring size of the analog and the unique conformational and structural character of the N-methylated amino-2-naphthoyl side chain of aminoglycine at position 8 and not in the Tyr7 substitution or in the d-configuration at position 3. The family of labeled and unlabeled sst3-ODN-8 analogs represents highly innovative, potent, and specific sst3-selective antagonist tools for the study of sst3-mediated physiological and pathophysiological conditions that may suggest novel clinical applications.
Somatostatin modulates numerous actions in the body (1, 2) that are mediated by at least five somatostatin receptor subtypes (3, 4). This large number of subtypes makes the understanding of the various receptor-mediated functions of somatostatin extremely difficult. Potent and subtype-selective antagonists are therefore needed to evaluate the physiological role of each subtype and may become important leads for drug discovery. Selective radioligands are also used to identify the tissue sites expressing the various receptors and may be used for both diagnostic and therapeutic indications. The secondary structure of such ligands (especially if constrained) can be determined by a number of techniques, including NMR in solution, and may be used to develop models of the ligand–receptor interactions.
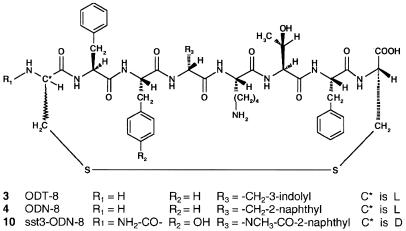
In the present study, we characterize both chemically and biologically several sst3-selective somatostatin-14 analogs (octapeptide derivatives cyclized via a disulfide bridge; see Fig. 1) identified from a betidamino acid scan of des-AA1, 2, 4, 5, 12, 13 [d-Trp8]-somatostatin-14 (ODT-8; ref. 5). Betidamino acids are N′-monoacylated (optionally, N′-monoacylated and N-mono- or N,N′-dialkylated) aminoglycine derivatives in which each N′-acyl/alkyl group may mimic naturally occurring amino acid side chains or introduce novel functionalities (6, 7).
Figure 1.
Generic structure of somatostatin octapeptide analogs.
We have tested members of this scan for their binding properties on cells transfected with the five human sst receptor subtypes. The most potent and selective analogs were then evaluated for their agonist/antagonist properties, using various bioassays, including inhibition of cAMP production and stimulation of phospholipase C (PLC). Moreover, sst3-transfected cells and human sst3-expressing tissues were labeled with the iodinated form of the best sst3-selective analogs.
Materials and Methods
Peptides.
The peptides described in this study are listed in Table 1. The two syntheses described below (A and B) of des-AA1, 2, 4, 5, 12, 13[d-Cys3,Tyr7,d-Agl8(Me,2-naphthoyl)]- somatostatin (compound 9; Fig. 1) are representative of that of all aminoglycine-containing peptide analogs presented here.
Table 1.
Chemical characteristics and sst1-5 binding affinity of sst3-selective and control peptides
| ID no. | Compound | Purity
|
MS
|
IC50,*
nM
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HPLC | CZE | Calc. | Found | sst 1 | sst 2 | sst 3 | sst 4 | sst 5 | ||
| 1 | SS-28 | >98 | >98 | 3147.5 | 3147.7 | 3.9 ± 0.3 (19) | 3.3 ± 0.2 (22) | 7.1 ± 0.7 (21) | 3.8 ± 0.3 (22) | 3.9 ± 0.3 (20) |
| 2 | Cortistatin | 96 | >98 | 1720.8 | 1720.9 | 3.9 ± 1.5 (4) | 4.6 ± 0.4 (3) | 6.8 ± 1.6 (3) | 2.5 ± 0.5 (3) | 13 ± 3.1 (4) |
| 3 | des-AA1,2,4,5,12,13[d-Trp8]-SS (ODT-8) | >94 | 98 | 1079.5 | 1079.2 | 28 ± 4.7 (3) | 44 ± 13 (4) | 13 ± 4 (3) | 1.3 ± 0.8 (3) | 45 ± 27 (3) |
| 4 | des-AA1,2,4,5,12,13[d-2Nal8]-SS (ODN-8) | >98 | 98 | 1090.5 | 1090.5 | 607 ± 167 (3) | 173 ± 41 (3) | 6.7 ± 1.9 (3) | 56 ± 22 (3) | 28 ± 15 (3) |
| 5 | des-AA1,2,4,5,12,13[dAgl8(2-naphthoyl)]-SS | >98 | >98 | 1119.5 | 1119.4 | >10,000 (4) | >10,000 (5) | >1,000 (4) | 165 ± 45 (3) | >10,000 (4) |
| 6 | des-AA1,2,4,5,12,13[d-Agl8(Me,2-naphthoyl)]-SS | 98 | >98 | 1133.5 | 1133.6 | >10,000 (4) | >10,000 (6) | 70 ± 17 (5) | >10,000 (3) | >10,000 (4) |
| 7 | des-AA1,2,4,5,12,13[d-Cys3, d-Agl8(Me,2-naphthoyl)]-SS | >98 | 98 | 1133.5 | 1133.5 | >10,000 (5) | >10,000 (4) | 115 ± 36 (4) | >10,000 (4) | >1,000 (4) |
| 8 | des-AA1,2,4,5,12,13[Tyr7, d-Agl8(Me,2-naphthoyl)]-SS | >98 | 98 | 1149.5 | 1149.2 | >10,000 (5) | >10,000 (5) | 70 ± 18 (4) | >1,000 (5) | >10,000 (4) |
| 9 | des-AA1,2,4,5,12,13[d-Cys3,Tyr7, d-Agl8(Me,2-naphthoyl)]-SS | >98 | >98 | 1149.5 | 1149.3 | >10,000 (7) | >10,000 (7) | 39 ± 11 (6) | >10,000 (7) | >10,000 (6) |
| 10 | Carbamoyl-des-AA1,2,4,5,12,13[d-Cys3, Tyr7, d-Agl8(Me,2-naphthoyl)]-SS (sst3-ODN-8) | >98 | — | 1192.4 | 1192.4 | >10,000 (3) | >10,000 (3) | 6.7 ± 2.6 (3) | >10,000 (3) | >10,000 (3) |
| 11 | L-796-778 | 585.7 | >10,000 (3) | >10,000 (3) | 127 ± 31 (4) | >10,000 (3) | >10,000 (3) | |||
SS, somatostatin-14; SS-28, somatostatin-28; MS, mass spectrum as [mass + H]+; CZE, capillary zone electrophoresis.
Mean ± SEM. Number of experiments in parentheses.
Compound 9 was synthesized (A) manually by the solid-phase approach, starting with Boc-Cys(pMeOBzl)-CM resin (1 g, 0.27 mmol/g). The Boc amino acids used in this synthesis were Boc-d-Cys(pMeOBzl)-OH, Boc-Phe-OH, Boc-Thr(Bzl)-OH, and Boc-Lys(2-Cl-Z)-OH. Couplings were mediated for 1 h by diisopropylcarbodiimide (DIC) or DIC with N-hydroxybenzotriazole (HOBt) in CH2Cl2 (DCM) and N-methylpyrrolidinone (NMP), respectively. A 3.0-equivalent excess of the protected amino acids based on the original substitution of the resin was used. TFA, 60% in DCM (2% m-cresol), was applied for 20 min to remove the Boc group. After coupling of the papain-resolved, optically active (R)-Boc,Fmoc-aminoglycine (8), the Boc group was removed and the Nα′-methylamino group of the aminoglycine was formed on the resin as described by Kaljuste (9). The naphthoyl side chain was introduced by its acid chloride to the free secondary amino group. Removal of the N-Fmoc protecting group of aminoglycine with 20% piperidine in NMP (2 × 10 min) allowed the elongation of the peptide backbone until completion. The peptide was cleaved and deprotected in HF (20 ml) in the presence of 10% anisole and 10% methyl sulfide for 90 min at 0°C. The diethyl ether-precipitated crude peptide was cyclized in 75% acetic acid (200 ml) by the addition of iodine (10% solution in methanol) until the appearance of a stable orange color. Forty minutes later, ascorbic acid was added to quench the excess of iodine. The lyophilized crude peptide (240 mg) was purified by preparative HPLC in two solvent systems (TEAP at pH 2.25 and 0.1% TFA, successively; ref. 10). The yield was 33 mg (see Table 1 for characterization).
The identical peptide was synthesized (B) with unresolved Boc-D/L-Agl(NMe,Fmoc)-OH (11), yielding two diastereomers separated by reversed-phase HPLC. The earlier eluting diastereomer obtained in synthesis B coeluted with the peptide described in synthesis A. They both showed the same mass, and their receptor binding affinity was identical.
Control peptides were somatostatin-28 (SS-28; Bachem, Switzerland), cortistatin, and ODT-8, all synthesized in our laboratory. L-796–778, a sst3-selective nonpeptidic analog, was a gift from Merck.
Cell Culture.
CHO-K1 cells stably expressing human sst1 and sst5 were kindly provided by Drs. T. Reisine and G. Singh (University of Pennsylvania, Philadelphia), and CCL39 cells stably expressing human sst2, sst3, and sst4 were provided by Dr. D. Hoyer (Novartis Pharma, Basel, Switzerland). Cells were grown as described previously (12). All culture reagents were supplied by GIBCO/BRL and Life Technologies (Grand Island, NY).
In Situ Hybridization Histochemistry.
To control the adequacy of the cell material, in situ hybridization for human sst mRNAs was performed on CHO-K1 and CCL39 cells expressing the different sst receptor subtypes. Cell smears were prepared, then used for sst1, sst2, sst3, sst4, and sst5 mRNA detection, as described in detail previously (12). These control in situ hybridization studies confirmed that the five cell lines used for the study expressed the correct sst mRNA.
Receptor Autoradiography.
Cell membrane pellets were prepared, and receptor autoradiography was performed on pellet sections (mounted on microscope slides), as described in detail previously (12). For each of the tested compounds, complete displacement experiments with the universal somatostatin radioligand 125I-[Leu8, d-Trp22, Tyr25]-somatostatin-28 (125I-[LTT]-somatostatin-28) (2,000 Ci/mmol; Anawa, Wangen, Switzerland), using 30,000 cpm/100 μl and increasing concentrations of the unlabeled peptide ranging from 0.1 to 1,000 nM, were performed. The unlabeled, universal somatostatin-28 was run as control in parallel, using the same increasing concentrations. IC50 values were calculated after quantification of the data with a computer-assisted image processing system as described previously (12, 13). Tissue standards (autoradiographic [125I] microscales; Amersham) that contain known amounts of isotope, cross-calibrated to tissue-equivalent ligand concentrations, were used for quantification (14). Advantages of the present method using receptor autoradiography with sectioned cell pellets compared with binding on cell homogenates are, in addition to an economic use of cells and a great flexibility, the greater interassay reliability and reproducibility, because the same embedded pellet can be used for successive experiments. A minor disadvantage is that IC50 values are somewhat higher than in the homogenate binding assay (12).
Three of the antagonists, compounds 8, 9, and 10 (Table 1), were iodinated at their Tyr7 position with the lactoperoxidase method and were HPLC purified (74 TBq/mmol; Anawa). Binding studies were performed with these ligands, as reported above with 125I-[LTT]-somatostatin-28, on cell pellet sections and on tissue sections of sst3-expressing human tumors, using 8,000 cpm/100 μl of the radioligand. Moreover, saturation experiments were performed using homogenates of the sst3-transfected cells and increasing amounts of 125I-labeled compound 10. They were performed as previously described (15), except that the incubation time with the radioligands was 1 h in a 10 mM Hepes buffer (pH 7.5) containing 10 mM MgCl2, bacitracin (40 μg/ml) and 0.1% BSA.
Adenylate Cyclase Activity.
Sst3-expressing cells were subcultured in 96-well culture plates at 2 × 104 cells per well and grown for 24 h. Culture medium was removed from the wells, and fresh medium (100 μl) containing 0.5 mM 3-isobutyl-I-methylxanthine (IBMX) was added to each well. Cells were incubated for 30 min at 37°C. Medium was then removed and replaced with fresh medium containing 0.5 mM IBMX, with or without 10 μM forskolin and various concentrations of peptides. Cells were incubated for 30 min at 37°C. After removal of the medium, cells were lysed and cAMP accumulation was determined using a commercially available cAMP scintillation proximity assay (SPA) system (RPA 538), according to the instructions of the manufacturer (Amersham Pharmacia). In these studies, basal levels of cAMP production were 0.6 ± 0.05 pmol cAMP per well, rising to 4.2 ± 0.3 pmol cAMP per well in the presence of 10 μM forskolin, representing a 7.7 ± 0.5 fold stimulation.
Phospholipase C Activity.
To measure phospholipase C activity, total [3H]inositol phosphate accumulation was determined with an anion exchange column assay as described in detail previously (16). Briefly, sst3-expressing cells were incubated in medium containing 2 μCi myo-[2-3H]inositol/ml (592 GBq/mmol; Amersham) for 24 h at 37°C. Cells were then washed and incubated for 5 min at 37°C in HBS buffer containing 20 mM LiCl to block inositol monophosphatase activity. Somatostatin-28 (10 μM) and various concentrations of antagonist were added, and the cells were incubated for another 50 min at 37°C. Cells were then extracted, and the extracts were applied to AGI-X8 columns (Bio-Rad). After elimination of free inositol and glycerophosphoinositol, total inositol phosphates (IPx) were eluted, and radioactivity was determined by liquid scintillation counting in a β counter. In these studies, basal levels of [3H]IPx production (in the absence of somatostatin-28) were 285 ± 25 cpm per well, rising to 742 ± 63 cpm per well in the presence of 10 μM somatostatin-28, representing a 2.6 ± 0.3–fold stimulation.
Analysis of Data.
cAMP and IPx data were expressed as percentages of stimulation over the nonstimulated level. Values of EC50 (the agonist concentration causing 50% of its maximal effect in the absence or presence of a fixed concentration of antagonist) and IC50 (the antagonist concentration reversing by 50% the effect of a fixed concentration of agonist) were derived from the concentration–response curves. The pKB values of antagonists were calculated according to the formula pKB = log [B] − log (CR-I), where [B] is the concentration of the antagonist used and CR (concentration ratio) is the ratio of agonist EC50 measured in the presence of antagonist over that measured in the absence of antagonist. The pKi values of antagonists were calculated, as the negative log10 of Ki, with the equation described by Cheng and Prusoff (17): Ki = IC50/[I + ([A]/EC50)], where [A] is the fixed agonist concentration.
Results
The structure of several sst3-selective and control peptides are shown in Fig. 1 and Table 1. Control peptides include somatostatin-28, cortistatin, ODT-8, ODN-8, and the sst3-selective nonpeptide L-796–778 reported by Rohrer et al. (18). Included in Table 1 are chromatographic and electrophoretic data describing the high level of purity of the analogs and their characterization by mass spectrometry. All peptides were tested in at least three separate experiments for their ability to bind to the five human cloned somatostatin receptors. Structures of compounds 5–10 are homologous to that of ODN-8. They are all cyclo(1–8)octapeptides with a mimetic of d-2-naphthylalanine at position 8. ODN-8 is homologous to the universal ligand ODT-8 in that the indole ring of d-Trp8 is replaced by a naphthyl ring. This substitution alone (compound 4) results in a significant loss of affinity for receptors sst1, sst2, and sst4 as compared with that of ODT-8 (compound 3). Replacing the d-2-naphthylalanine in ODN-8 with the corresponding betidenaphthylalanine [Agl(2-naphthoyl)] (6) as in 5 resulted in a loss of binding affinity (IC50 > 1,000 nM) for all receptors but sst4 (IC50 = 165 nM). Methylating the side chain of betidenaphthylalanine as in 6, however, restored affinity for sst3 and increased selectivity considerably (>100-fold). Introduction of a d-Cys residue at position 3 had no influence on either affinity or sst3 selectivity if we compare the binding properties of 6 with those of 7. Introduction of a Tyr residue at position 7 had no influence on either affinity or selectivity if we compare the binding properties of 6 with those of 8. The introduction of both d-Cys at position 3 and Tyr at position 7 in peptide 6 resulted in 9, with increased binding affinity (IC50 = 39 nM) and selectivity for the receptor sst3 (IC50 > 10,000 nM for all other somatostatin receptors). Finally, the acylation of the N terminus of 9 with a carbamoyl group yielded the most potent and sst3-selective compound of this series, sst3-ODN-8 (compound 10), with an sst3 affinity equal to that of somatostatin-28. All l-Agl8 isomers of compounds 5–10 were inactive on all five receptor subtypes (IC50 > 1,000 nM). The sst3-selective nonpeptide L-796–778 was found in our assays to have an IC50 = 127 nM for sst3 and greater than 10,000 for all other receptors.
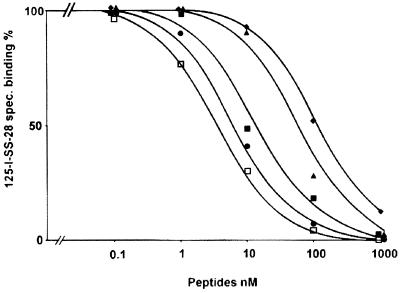
Whereas in our system our best analog, 10, is as potent as somatostatin-28, L-796–778 is 18 times less potent than somatostatin-28 at sst3; both compounds, however, are highly selective for sst3. A representative example of displacement experiments using sst3-expressing cells is shown in Fig. 2.
Figure 2.
Competition experiment in sst3-transfected CCL 39 cells, using 125I-[LTT]-SS-28 as a radioligand and increasing concentrations of somatostatin-28 (●) and ODT-8 (■), as well as the three sst3-selective analogs 6 (♦), 9 (▴), and 10 (□).
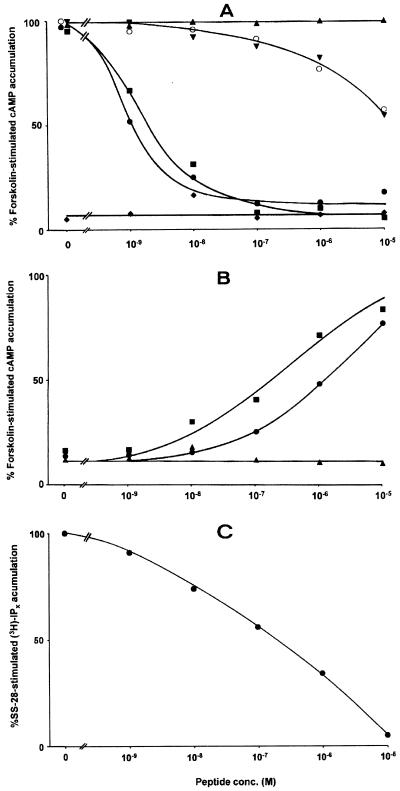
To distinguish agonists from antagonists, the effects of the best sst3-selective analogs on forskolin-stimulated cAMP production in sst3-expressing CCL39 cells were evaluated. Table 2 summarizes the data. The two agonists somatostatin-28 and ODT-8 potently inhibited forskolin-stimulated cAMP accumulation by more than 87% (87.8% and 94.2%, respectively) at a peptide concentration of 100 nM, with an EC50 of approximately 1 nM (Fig. 3); they were used as controls. Compounds 6–10 given alone could not inhibit cAMP production. However, the agonistic effect of somatostatin-28 could be massively antagonized with a fixed concentration of 10−5 M for each of the sst3-selective analogs (Table 2, Fig. 3A). pKB values of 9.04 and 9.07 were calculated for the two best analogs, compounds 9 and 10, respectively. Furthermore, the dose-dependent decrease in cAMP accumulation by SS-28 in the presence of increasing concentrations of antagonists yielded a series of rightward shifted dose–response curves. The rank order of potency of antagonist action was comparable to the rank order of potency of binding of the tested analogs. Conversely, the inhibition of the forskolin-stimulated cAMP production by 5 × 10−8 M somatostatin-28 could be reversed completely and dose-dependently by increasing concentrations of the sst3-selective analogs (Table 2, Fig. 3B). Again, the rank order of antagonist efficiency was the same as the rank order of binding affinity. Therefore, all five analogs are competitive antagonists at the sst3 receptor, with 10 being the most potent.
Table 2.
Agonist and antagonist properties of selective analogs on cAMP accumulation in sst3 cells
| ID no. | Name | Effect on cAMP accumulation
|
||||
|---|---|---|---|---|---|---|
| Agonists
|
Antagonists
|
|||||
| A
|
B
|
C
|
||||
| EC50, nM | EC50, nM | pKB | IC50, nM | pKi | ||
| 1 | SS-28 | 0.87 ± 0.17 | — | — | — | — |
| 3 | ODT-8 | 1.31 ± 0.13 | — | — | — | — |
| 10 | sst3-ODN-8 | — | 10,230 ± 1,450 | 9.07 | 335 ± 250 | 8.24 |
| 9 | — | 9,410 ± 290 | 9.04 | 430 ± 170 | 8.13 | |
| 8 | — | 980 ± 310 | 8.05 | 960 ± 315 | 7.78 | |
| 6 | — | 840 ± 150 | 7.99 | 1,510 ± 230 | 7.59 | |
| 7 | — | 650 ± 90 | 7.88 | 2,040 ± 720 | 7.46 | |
A, Agonist alone; B, SS-28 versus 10−5 M antagonist; C, antagonist versus 5 × 10−8 M SS-28. All values are mean ± SE (n = 3).
Figure 3.
(A) Effects of various concentrations of somatostatin-28 and analogs on forskolin-stimulated cAMP accumulation in cells expressing sst3. Concentration–response curves were obtained with increasing concentrations of somatostatin-28 (●), ODT-8 (■) or 9 (▴), and of somatostatin-28 in the presence of 10−5 M 9 (▾),10−5 M 10 (○), or 10−5 M ODT-8 (♦). Data are expressed as a percentage of the 10 μM forskolin response. The somatostatin analogs 9 and 10 antagonize the concentration-dependent inhibition of cAMP accumulation by somatostatin-28 in sst3-expressing cells. (B) Effects of various concentrations of somatostatin analogs in the presence of a fixed concentration of somatostatin-28 on forskolin-stimulated cAMP accumulation in cells expressing sst3. Concentration–response curves were obtained with increasing concentrations of 7 (●), 10 (■), and ODT-8 (▴) in the presence of 5 × 10−8 M somatostatin-28. Data are expressed as a percentage of the 10 μM forskolin response. The somatostatin analogs 7 and 10 concentration-dependently reverse the inhibition of cAMP accumulation by somatostatin-28 in sst3-expressing cells. (C) Effects of various concentrations of 10 on somatostatin-28-stimulated IPx accumulation in cells expressing sst3. The concentration–response curve was obtained with increasing concentrations of 10 in the presence of 10−5 M somatostatin-28. Data are expressed as a percentage of the 10 μM somatostatin-28 response. 10 antagonizes concentration-dependently the stimulation of IPx accumulation by somatostatin-28 in sst3-expressing cells.
We further evaluated the effect of 9 and 10 on phospholipase C activity in sst3-expressing cells (Fig. 3C). Whereas somatostatin-28 strongly stimulated the PLC activity, this effect could be reversed completely by 9 and 10 with an IC50 of 275 nM ± 109 (mean ± SE, n = 3) and 166 nM ± 20 (mean ± SE, n = 3), respectively. Given alone, however, 9 and 10 had no effect on PLC activity. These data confirm, therefore, the competitive antagonist properties of the compounds. The pKi of these compounds was calculated to be 9.00 and 9.22, respectively, using 36.5 nM as the EC50 value for somatostatin-28, a value that is close to the one reported in the literature (16).
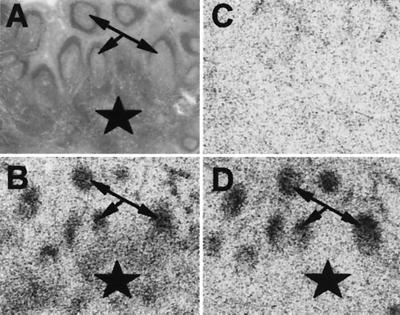
As compounds 8–10 have a Tyr substitution at position 7, they could be radioiodinated and used as radioligands. In preliminary experiments we could show that compound 10 retained its sst3-binding affinity and sst3 selectivity when iodinated with 127I at position 7 [IC50 for sst3: 18.6 ± 5.8 nM (mean ± SEM; n = 4); IC50 for sst1, sst2, sst4, and sst5: >10,000 nM (n = 4)]. All three antagonists, when 125I-labeled, were able to strongly and selectively label sst3-transfected CCL39 cells. An example is shown in Fig. 4, using monoiodinated compound 10. Moreover, saturation experiments using the same ligand in sst3-expressing cell homogenates and Scatchard analysis of the data revealed a high-affinity binding with a KD of 0.27 nM (data not shown). Furthermore, we used the radioiodinated compounds 8–10 to try to label selected human tumors known to express sst3. Whereas compounds 8 and 9 had strong nonspecific binding and did not allow the identification of specific sst3-binding sites in the tumors, we were able to label with iodinated compound 10 several human tumors belonging to the group of inactive pituitary adenomas, selected on the basis of their abundance of sst3 mRNA, as shown with in situ hybridization techniques. An example of one inactive pituitary adenoma is shown in Fig. 4. Specific high-affinity binding of 125I-10 was clearly identifiable, despite the presence of a significant nonspecific background binding. In nonneoplastic normal rat or human tissues, where sst3 receptors may be expressed in a much lower density than in tumors (19, 20), the use of 125I-10 did not permit the visualization of specific sites. However, by using the universal ligand 125I-[LTT]-somatostatin-28 as a tracer, it was possible to specifically displace sst3-specific binding sites with nanomolar concentrations of compound 10 in selected human somatostatin target tissues such as T-cell-rich interfollicular areas of the lymphoreticular system in the tonsils (Fig. 5) and lymph nodes.
Figure 4.
(Left) Binding of 125I-10 to sections containing sst1-sst5-expressing cell pellets. All sections were incubated with 8,000 cpm/100 μl of ligand. Strong specific labeling with the ligand is seen only in sst3 cells. (A, C, E, G, I) Autoradiograms showing total binding of 125I-10 to sections of cell pellets expressing sst1 (A), sst2 (C), sst3 (E), sst4 (G), and sst5 (I). (B, D, F, H, K) Autoradiograms showing nonspecific binding of 125I-10 (in the presence of 10−6 M unlabeled 10). (Right) In vitro receptor autoradiography detection of sst3 receptors in a human inactive pituitary adenoma. (A) Hematoxylin and eosin-stained section. Bar = 1 mm. (B) Autoradiogram showing total binding of 125I-labeled 10. (C) Autoradiogram showing nonspecific binding of 125I-10 (in the presence of 10−6 M unlabeled 10). The tumor was shown to have predominantly and abundantly sst3 mRNA with in situ hybridization. Although a significant nonspecific binding is seen in C, specific binding can nevertheless be clearly identified.
Figure 5.
sst3 in human lymphoreticular tissue. (A) Hematoxylin-eosin-stained section showing parts of a human tonsil containing a T-cell-rich interfollicular area (around the asterisk) and B-cell-rich germinal centers (arrows). (B) Autoradiogram showing total binding of 125I-[LTT]-somatostatin-28, with labeling of the interfollicular area (around the asterisk) and of germinal centers (arrows). (C) Autoradiogram showing nonspecific binding of 125I-[LTT]-somatostatin-28 (in the presence of 10−6 M somatostatin-28). (D) Autoradiogram showing binding of 125I-[LTT]-somatostatin-28 in the presence of 10−7 M compound 10. Binding to the interfollicular area is abolished, strongly indicating sst3 expression in this area. Binding to the germinal centers remains; they are known to express sst2 (43).
Discussion
The present study describes a family of potent peptidic analogs of somatostatin with competitive antagonistic properties and high selectivity for the sst3 receptor subtype. With its high sst3 affinity, comparable to that of the natural somatostatin-28, the best compound, 10 (sst3-ODN-8), is significantly better than any other sst3-selective analog, including the recently discovered nonpeptide agonist L-796–778 reported by Rohrer et al. (18). Analog 10 also shows a higher sst3 selectivity than the novel, partially selective antagonists recently reported by Hocart (21). Furthermore, 10 is a potent antagonist of somatostatin-28 and ODT-8, as shown in cAMP assays as well as in PLC assays, with no measurable intrinsic activity at the highest dose tested.
As described under Results, the selectivity of 10 is dependent on the octapeptide scaffold and critically on the introduction of a methyl group on the nitrogen side chain of d-Agl at position 8, inasmuch as neither the dCys3 substitution in 7 nor the Tyr7 substitution in 8 alters the sst3 selectivity discovered in 6. From earlier modeling studies (6) it was clear that methylation of the side-chain amino function of aminoglycine would play a significant role in constraining side-chain mobility. Further NMR investigations are needed to identify the conformational space occupied by this side chain as compared with that occupied by the corresponding side chain in parent analogs.
The peptidic nature of our sst3 antagonists results in a number of advantageous properties: (i) Their structures can be modified easily and rationally to obtain more potent or more stable compounds. (ii) The relatively large molecules allow for the addition of further isotopes or even chelators with isotopes without significant modification of their binding affinity. Labeling of such compounds is of great interest, because it allows researchers to obtain 125I-labeled radioligands as tools for the tissue localization of sst3 sites or to design chelator-linked analogs to which 99Tc, 111In, 188Re, or 90Y could be attached. Octreotide-based peptides linked to such isotopes are currently used in nuclear medicine for diagnostic or therapeutic purposes (22, 23). (iii) A peptide will poorly cross the blood–brain barrier. If it is to be used for systemic application as a drug or radioligand in clinical indications involving peripheral tissues and tumors, it will be a great advantage if it does not penetrate into the brain, an organ known to contain sst3. Otherwise, it may interact with these sst3 receptors and radiolabel the brain in vivo (as a radioligand) or have functional side effects (as a nonradioactive drug) in the central nervous system.
These selective sst3 antagonists are tools for searching for the localization of sst3 in human tissues and deciphering the functions mediated by sst3. Currently, the sst3 system is poorly understood, specifically in humans. If we want to take advantage of sst3-selective analogs to affect sst3-mediated functions clinically, a better knowledge of this system is mandatory, at both the physiological and pathophysiological levels. Currently available information on sst3 includes the observation that sst3 is present in the human brain, where somatostatin may play a neurotransmitter role (19, 24), and that sst3 is also found in the smooth muscles of the guinea pig and rat gastrointestinal tract, where they may mediate muscular contractility (20, 25, 26).
Furthermore, sst3 may play a role in cancer. The sst3 mRNA can be detected in various types of human tumors (27–29). However, the incidence and importance of sst3 in human tumors is still a matter of debate. According to Virgolini et al. (30), sst3 is the most frequently expressed somatostatin receptor, including in exocrine pancreatic cancers; according to the majority of other authors (31–34), it is relatively rarely expressed in tumors. Because most of these studies were performed by measuring sst3 mRNA, it is evident that a confirmation of the presence or absence of the receptor protein, using sensitive receptor binding assays with selective radioligands such as 125I-10, would be welcome, as no reports using sst3 antibodies have conclusively answered this question. We can show here that inactive pituitary adenomas often express sst3.
According to some authors (35, 36), the somatostatin effect mediated by sst3 in tumors may possibly be a particularly important one, as it may directly regulate cellular growth processes. It was suggested by Yoshitomi et al. (35) that in the mouse insulinoma MIN6 cells sst3 may mediate the somatostatin inhibition of cell growth. Buscail et al. (31) have seen, however, no effect of the somatostatin analog RC-160 on the growth of sst3-transfected CHO cells. Furthermore, the somatostatin stimulation of protein tyrosine phosphatase activity, an activation associated with somatostatin inhibition of cell proliferation (37, 38), and the ensuing protein tyrosine phosphatase-dependent inactivation of Raf-1, were shown to be mediated by sst3 transiently expressed in Ras-transformed NIH 3T3 cells by Reardon et al. (39, 40). On the other hand, somatostatin has been shown to increase the proliferation rate of mitogen-activated human leukemia T (Jurkat) cells—through increase of IL-2 secretion—via the single receptor subtype sst3 expressed by these cells (41). Sharma et al. (36) have suggested that somatostatin may stimulate apoptosis through sst3 mediation. A better understanding of the involved mechanisms of action on tumor growth regulation may become possible with the use of selective sst3 antagonists. As a step toward answering all of these questions, we have already shown that we can identify sst3 receptors on certain tumors.
The labeled and unlabeled members of the present family of sst3 antagonists represent an important step toward the identification of potential tools for clinical applications in humans. Labeling of 9 with 111In or 99Tc linked to a chelator may yield a radioligand suitable for the in vivo scintigraphy of sst3-expressing tumors, which are presently poorly identified with Octreoscan (22), because of its relatively lower affinity for sst3. Moreover, 90Y labeling of this antagonist may yield a radiotherapeutic agent to destroy sst3-expressing tumors, as appears to occur with sst2 tumors when 90Y-DOTATOC is used (23). For such indications, we need to explore whether and the extent to which sst3 antagonists can be internalized; although the general belief is that antagonists poorly internalize, it was convincingly shown recently that cholecystokinin-B receptor antagonists can (42). A drug based on sst3 antagonism may also be clinically useful in nononcological fields. The use of sst3-ODN-8 in the treatment of gastrointestinal diseases, in particular gut motility disorders, should be worth investigating once it can be confirmed that sst3 plays a major role in the human gastrointestinal smooth muscle contraction (25, 26). Moreover, the presence of sst3 in the human lymphoreticular tissue, as shown in the present study, may suggest the use of sst3-ODN-8 for the treatment of certain immune diseases. Most of these studies are being contemplated.
Acknowledgments
We thank Dr. D. Hoyer (Novartis, Basel) and Drs. T. Reisine and G. Singh (University of Pennsylvania, Philadelphia) for the generous gift of sst-transfected cells. We are indebted to R. Galyean, W. Low, D. Kirby, R. Kaiser, and T. Goedken for technical assistance in the synthesis and characterization of the peptides and to Dr. A G. Craig for mass spectrometric analysis of the analogs. We also thank Merck for the gift of L-796-778. This work was supported in part by Grant 5ROI DK 50124 from the National Institutes of Health and the Hearst Foundation.
Abbreviations
- ODN-8
des-AA1,2,4,5,12,13[d-2Nal8]-somatostatin-14
- sst3-ODN-8
carbamoyl-des-AA1,2,4,5,12,13[d-Cys3,Tyr7,d-Agl8(Me,2-naphthoyl)]-somatostatin-14
- ODT-8
des-AA1,2,4,5,12,13[d-Trp8]-somatostatin-14
- 125I-[LTT]-somatostatin-28
125I-[Leu8, d-Trp22, Tyr25]-somatostatin-28
- PLC
phospholipase C
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250483897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250483897
References
- 1.Reichlin S. N Engl J Med. 1983;309:1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- 2.Reichlin S. N Engl J Med. 1983;309:1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- 3.Reisine T, Bell G I. Endocr Rev. 1995;16:427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer D, Bell G I, Berelowitz M, Epelbaum J, Feniuk W, Humphrey P P A, O'Carroll A, Patel Y C, Schonbrunn A, Taylor J E, et al. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 5.Vale W, Rivier C, Brown M, Rivier J. In: Hypothalamic Peptide Hormones and Pituitary Regulation: Advances in Experimental Medicine and Biology. Porter J C, editor. New York: Plenum; 1977. pp. 123–156. [Google Scholar]
- 6.Rivier J E, Jiang G-C, Koerber S C, Porter J, Craig A G, Hoeger C. Proc Natl Acad Sci USA. 1996;93:2031–2036. doi: 10.1073/pnas.93.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang G, Miller C, Koerber S C, Porter J, Craig A G, Bhattacharjee S, Kraft P, Burris T P, Campen C A, Rivier C L, et al. J Med Chem. 1997;40:3739–3748. doi: 10.1021/jm970024p. [DOI] [PubMed] [Google Scholar]
- 8.Sypniewski M, Penke B, Simon L, Rivier J. J Org Chem. 2000;65:6595–6600. doi: 10.1021/jo000736e. [DOI] [PubMed] [Google Scholar]
- 9.Kaljuste K, Unden A. Int J Pept Protein Res. 1993;42:118–124. doi: 10.1111/j.1399-3011.1993.tb00487.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller C, Rivier J. Biopolymers. 1996;40:265–317. doi: 10.1002/(SICI)1097-0282(1996)40:3%3C265::AID-BIP2%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Jiang G-C, Simon L, Rivier J E. Protein Pept Lett. 1996;3:219–224. [Google Scholar]
- 12.Reubi J C, Schaer J C, Waser B, Wenger S, Heppeler A, Schmitt J, Mäcke H R. Eur J Nucl Med. 2000;27:273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 13.Reubi J C, Kvols L K, Waser B, Nagorney D, Heitz P U, Charboneau J W, Reading C C, Moertel C. Cancer Res. 1990;50:5969–5977. [PubMed] [Google Scholar]
- 14.Reubi J C. J Nucl Med. 1995;36:1846–1853. [PubMed] [Google Scholar]
- 15.Reubi J C, Maurer R, Klijn J G M, Stefanko S Z, Foekens J A, Blaauw G, Blankenstein M A, Lamberts S W J. J Clin Endocrinol Metab. 1986;63:433–438. doi: 10.1210/jcem-63-2-433. [DOI] [PubMed] [Google Scholar]
- 16.Siehler S, Hoyer D. Naunyn-Schmiedeberg's Arch Pharmacol. 1999;360:522–532. doi: 10.1007/s002109900144. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Prusoff W H. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 18.Rohrer S P, Birzin E T, Mosley R T, Berk S C, Hutchins S M, Shen D, Xiong Y, Hayes E C, Parmar R M, Foor F, et al. Science. 1998;282:737–740. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- 19.Hervieu G, Emson P C. Mol Brain Res. 1999;71:290–303. doi: 10.1016/s0169-328x(99)00201-6. [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Corleto V D, Mantey S A, Coy D H, Maton P N, Jensen R T. Am J Physiol. 1995;268:G739–G748. doi: 10.1152/ajpgi.1995.268.5.G739. [DOI] [PubMed] [Google Scholar]
- 21.Hocart S J, Jain R, Murphy W A, Taylor J E, Coy D H. J Med Chem. 1999;42:1863–1871. doi: 10.1021/jm9806289. [DOI] [PubMed] [Google Scholar]
- 22.Krenning E P, Kwekkeboom D J, Pauwels S, Kvols L K, Reubi J C. In: Somatostatin Receptor Scintigraphy. Freeman L M, editor. New York: Raven; 1995. pp. 1–50. [Google Scholar]
- 23.Otte A, Mueller-Brand J, Dellas S, Nitzsche E U, Herrmann R, Maecke H R. Lancet. 1998;351:417–418. doi: 10.1016/s0140-6736(05)78355-0. [DOI] [PubMed] [Google Scholar]
- 24.Händel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Höllt V. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 25.Corleto V D, Weber H C, Jensen R T. Am J Physiol. 1999;277:G235–G244. doi: 10.1152/ajpgi.1999.277.1.G235. [DOI] [PubMed] [Google Scholar]
- 26.Murthy K S, Coy D H, Makhlouf G M. J Biol Chem. 1996;271:23458–23463. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- 27.Reubi J C, Schaer J C, Waser B, Mengod G. Cancer Res. 1994;54:3455–3459. [PubMed] [Google Scholar]
- 28.Schaer J C, Waser B, Mengod G, Reubi J C. Int J Cancer. 1997;70:530–537. doi: 10.1002/(sici)1097-0215(19970304)70:5<530::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 29.Greenman Y, Melmed S. J Clin Endocrinol Metab. 1994;78:398–403. doi: 10.1210/jcem.78.2.8106629. [DOI] [PubMed] [Google Scholar]
- 30.Virgolini I. Eur J Clin Invest. 1997;27:793–800. doi: 10.1046/j.1365-2362.1997.1990742.x. [DOI] [PubMed] [Google Scholar]
- 31.Buscail L, Saint-Laurent N, Chastre E, Vaillant J C, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C. Cancer Res. 1996;56:1823–1827. [PubMed] [Google Scholar]
- 32.Fisher W E, Doran T A, Muscarella P, II, Boros L G, Ellison E C, Schirmer W J. J Natl Cancer Inst. 1998;90:322–324. doi: 10.1093/jnci/90.4.322. [DOI] [PubMed] [Google Scholar]
- 33.Sinisi A A, Bellastella A, Prezioso D, Nicchio M R, Lotti T, Salvatore M, Pasquali D. J Clin Endocrinol Metab. 1997;82:2566–2569. doi: 10.1210/jcem.82.8.4142. [DOI] [PubMed] [Google Scholar]
- 34.Miller G M, Alexander J M, Bikkal H A, Katznelson L, Zervas N T, Klibanski A. J Clin Endocrinol Metab. 1995;80:1386–1392. doi: 10.1210/jcem.80.4.7714115. [DOI] [PubMed] [Google Scholar]
- 35.Yoshitomi H, Fujii Y, Miyazaki M, Nakajima N, Inagaki N, Seino S. Am J Physiol. 1997;272:E769–E774. doi: 10.1152/ajpendo.1997.272.5.E769. [DOI] [PubMed] [Google Scholar]
- 36.Sharma K, Patel Y C, Srikant C B. Mol Endocrinol. 1996;10:1688–1696. doi: 10.1210/mend.10.12.8961277. [DOI] [PubMed] [Google Scholar]
- 37.Buscail L, Delesque N, Estève J, Saint-Laurent N, Prats H, Clerc P, Robberecht P, Bell G I, Liebow C, Schally A V, et al. Proc Natl Acad Sci USA. 1994;91:2315–2319. doi: 10.1073/pnas.91.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buscail L, Estève J, Saint-Laurent N, Bertrand V, Reisine T, O'Carroll A, Bell G I, Schally A V, Vaysse N, Susini C. Proc Natl Acad Sci USA. 1995;92:1580–1584. doi: 10.1073/pnas.92.5.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reardon D B, Wood S L, Brautigan D L, Bell G I, Dent P, Sturgill T W. Biochem J. 1996;314:401–404. doi: 10.1042/bj3140401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reardon D B, Dent P, Wood S L, Kong T, Sturgill T W. Mol Endocrinol. 1997;11:1062–1069. doi: 10.1210/mend.11.8.9960. [DOI] [PubMed] [Google Scholar]
- 41.Cardoso A, El Ghamrawy C, Gautron J-P, Horvat B, Gautier N, Enjalbert A, Krantic S. J Cell Biochem. 1998;68:62–73. doi: 10.1002/(sici)1097-4644(19980101)68:1<62::aid-jcb6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Roettger B F, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, Miller L J. Mol Pharmacol. 1997;51:357–362. [PubMed] [Google Scholar]
- 43.Reubi J C, Laissue J A, Waser B, Steffen D L, Hipkin R W, Schonbrunn A. J Clin Endocrinol Metab. 1999;84:2942–2950. doi: 10.1210/jcem.84.8.5878. [DOI] [PubMed] [Google Scholar]