Abstract
Objective: To determine by modern echocardiographic techniques the prevalence and development of cardiac abnormalities associated with ventricular septal defect (VSD).
Methods: Consecutive patients referred to a tertiary centre for paediatric cardiology and attenders at an adult congenital heart disease clinic had details of clinical outcome prospectively recorded. Patients with VSD in association with conotruncal abnormalities, atrioventricular septal defects, and univentricular heart were not included in the study.
Results: 1448 patients with VSD were assessed between 1991 and 1998. 1127 (78%) patients had isolated defects; of these 862 (76%) were perimembranous and 265 (24%) were muscular. Of the remainder, 284 (19.6% of the total population) patients had VSD associated with one other significant cardiac abnormality: 35 (2.4%) with two and two (0.1%) with three other abnormalities. The most common associated cardiac abnormalities were infundibular pulmonary stenosis (5.8%), aortic valve prolapse (3.6%), pulmonary valvar stenosis (2.7%), osteum secundum atrial septal defect (2.2%), persistent ductus arteriosus (1.9%), and coarctation of the aorta (1.5%). In the cohort of 743 patients followed up from birth, cumulative mortality was 4% by the age of 8 years and most deaths occurred within the first year of life in infants with associated genetic abnormalities such as trisomy 13 or 18. Of the 594 patients attending the adult congenital clinic with VSD, aortic regurgitation due to aortic root prolapse developed in 45 (7.6%) patients. Most of these cases were detected before the patient was 30 years old.
Conclusions: In 22% of patients with congenital VSD there were significant associated cardiac abnormalities. Some of these abnormalities, such as aortic prolapse and regurgitation or infundibular pulmonary stenosis, may develop or progress subsequently and therefore should be sought during the initial assessment and monitored during follow up. Follow up to the age of 30 years allows the detection of most cases of aortic regurgitation. The prognosis from VSD is excellent and the risk of endocarditis in a population informed of the need for antibiotic prophylaxis is small.
Keywords: ventricular septal defect, echocardiography, aortic regurgitation: congenital heart disease
Ventricular septal defects (VSDs) are the most common group of congenital cardiac malformations and occur in 2–6 in 1000 live births.1,2 The pattern of defect can range from a tiny hole in the muscular septum, with a good chance of spontaneous closure, to complicated lesions requiring open heart surgery.3–6 The prognosis of patients with VSD is good; a large study of patients with congenital VSD found 87% survival at 25 years although there was a small but significant risk of serious arrhythmia and sudden death even among patients with small defects.6 Left ventricular function in some patients may be impaired although the mechanism involved is uncertain.7
The prevalence of additional cardiac malformations has been defined in selected groups of patients. In 156 infants with defects large enough to consider cardiac surgery, investigation by echocardiography and cardiac catheterisation confirmed the presence of infundibular pulmonary stenosis in 25%, subaortic ridges in 3%, and persistent ductus arteriosus in 15%.8 A study of 207 patients with muscular VSD found an association with coarctation of the aorta in 11 patients (5%).4 Studies of other cardiac abnormalities, such as persistent ductus arteriosus, confirmed the coexistence of VSD (eight of 146 patients in one group, 5%).9 The presence or development of such abnormalities may necessitate cardiac surgery or long term follow up.
The prevalence of additional abnormalities identified by modern ultrasound technology including colour flow Doppler has not been examined prospectively in a large unselected population. Transthoracic echocardiography with Doppler colour flow mapping is a sensitive and specific technique for the confirmation of clinically suspected VSD2,8,10 and the delineation of associated structural abnormalities.
The aim of this study was to examine the prevalence and characteristics of cardiac abnormalities in patients with congenital VSD by using modern echocardiographic techniques and to identify any further structural abnormalities that may develop with age. Patients with conotruncal abnormalities (transposition of the great arteries, tetralogy of Fallot, persistent truncus arteriosus, or double outlet right ventricle), univentricular heart, and atrioventricular septal defects have a different clinical entity with higher morbidity, mortality, and a corresponding need for corrective surgery. They have not been included in this study.
METHODS
Details were prospectively recorded for consecutive patients referred to a regional centre for paediatric cardiology or associated satellite clinics who were found to have congenital VSD. VSD was diagnosed by clinical examination and confirmed by transthoracic echocardiography including Doppler colour flow mapping. Additional cardiac abnormalities were sought in these patients and recorded if present.
In addition, patients followed up at an adult congenital heart disease clinic with a known diagnosis of VSD were identified.
Subsequent follow up visits were recorded, as well as details of clinical events including the need for cardiac surgery and cardiovascular complications such as endocarditis and death.
Pulmonary valvar stenosis was diagnosed by echocardiography according to conventional criteria and classified as mild (<25 mm Hg), moderate (25–49 mm Hg), or severe (> 49 mm Hg) based on estimated pressure gradients calculated by measurement of flow velocities across the valve.11 Infundibular pulmonary stenosis was graded in severity by the same criteria.
Aortic valve prolapse was diagnosed when a deformed aortic valve pivoted from the crest of the interventricular septum, noticeable in the parasternal long axis view.12 The degree of aortic regurgitation was defined as trivial if the colour flow regurgitation jet was limited to just below the aortic valve, mild if it did not quite reach the mitral valve tip, moderate if it reached the mitral valve tip, and severe if it was beyond the mitral valve tip.13
Statistical analysis
The χ2 test was used for frequency comparisons between groups and was applied only where more than 20% of the expected values in the contingency table are greater than 5. Percentages shown are of the total study population unless otherwise specified. We used SPSS software (version 9.0, SPSS Inc, Chicago, Illinois, USA).
RESULTS
Between 1991 and 1998, 1448 patients with congenital VSD were identified and assessed by echocardiography. Figure 1 shows the age distribution, which is clearly bimodal, representative of the two patient populations (paediatric and adult groups) studied.
Figure 1.
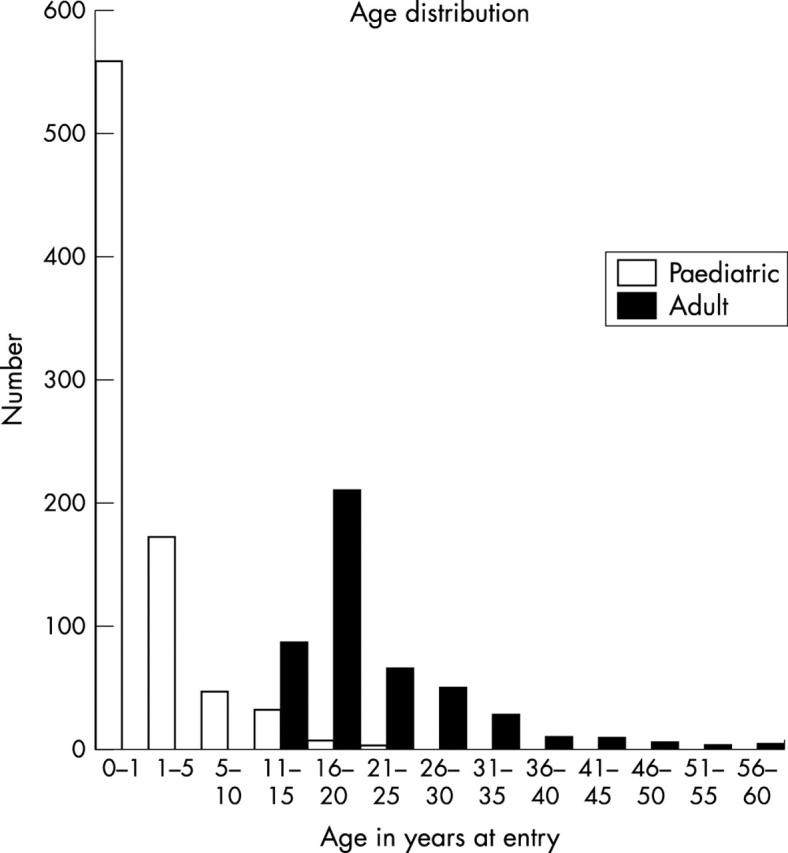
Age distribution of 1448 patients with congenital ventricular septal defect.
Associated cardiac defects
Table 1 shows additional associated cardiac defects in order of frequency for new paediatric referrals and adults seen at the adult congenital clinic. When all patients were considered together the most commonly associated cardiac abnormalities were infundibular pulmonary stenosis (5.8%), aortic prolapse (3.6%), pulmonary valvar stenosis (2.7%), osteum secundum atrial septal defect (2.2%), persistent ductus arteriosus (1.9%), and coarctation of the aorta (1.5%). The latter three defects listed were significantly less frequent in the adult group because most had been corrected in childhood by surgery or percutaneous intervention. Aortic valve prolapse was more frequent in the adult group (6.7% v 1.3%, p < 0.0001), as was infundibular pulmonary stenosis (7.8% v 4.3%, p < 0.005).
Table 1.
Prevalence of additional cardiac abnormalities in patients with ventricular septal defect (VSD) with a comparison of frequencies between the adult and paediatric groups
| Group | p Value | |||
| All | Paediatric | Adult | ||
| IPS | 84 (5.80%) | 36 (4.31%) | 48 (7.84%) | <0.005 |
| Aortic prolapse | 52 (3.59%) | 11 (1.32%) | 41 (6.70%) | <0.0001 |
| Pulmonary valvar stenosis | 39 (2.69%) | 25 (2.99%) | 14 (2.29%) | NS |
| Osteum secundum ASD | 32 (2.21%) | 23 (2.75%) | 9 (1.47%) | NS |
| PDA | 27 (1.86%) | 24 (2.87%) | 3 (0.49%) | <0.001 |
| CoA | 22 (1.52%) | 20 (2.39%) | 2 (0.33%) | <0.005 |
| Osteum secundum ASD and PDA | 13 (0.90%) | 12 (1.44%) | 1 (0.16%) | <0.05 |
| Mitral regurgitation | 12 (0.83%) | 5 (0.60%) | 7 (1.14%) | NS |
| IPS and aortic prolapse | 8 (0.55%) | 3 (0.36%) | 5 (0.82%) | NS |
| Wolff-Parkinson-White syndrome | 5 (0.35%) | 3 (0.36%) | 2 (0.33%) | NS |
| Aortic valvar stenosis | 3 (0.21%) | 2 (0.24%) | 1 (0.16%) | NS |
| Pulmonary valvar stenosis and PDA | 3 (0.21%) | 3 (0.36%) | 0 | NA |
| PBS | 3 (0.21%) | 0 | 3 (0.49%) | NA |
| CoA and IPS | 2 (0.14%) | 2 (0.24%) | 0 | NA |
| Aortic valvar stenosis and CoA | 2 (0.14%) | 2 (0.24%) | 0 | NA |
| IPS and osteum secundum ASD | 2 (0.14%) | 1 (0.12%) | 1 (0.16%) | NA |
| PBS and ASD | 2 (0.14%) | 1 (0.12%) | 1 (0.16%) | NA |
| Mitral stenosis | 2 (0.14%) | 0 | 2 (0.33%) | NA |
| Dextrocardia | 2 (0.14%) | 0 | 2 (0.33%) | NA |
| Aortic prolapse and IPS and CoA | 1 (0.07%) | 1 (0.12%) | 0 | NA |
| Pulmonary supravalvar stenosis | 1 (0.07%) | 1 (0.12%) | 0 | NA |
| Aortic valvar stenosis and PDA | 1 (0.07%) | 1 (0.12%) | 0 | NA |
| Mitral stenosis and CoA | 1 (0.07%) | 1 (0.12%) | 0 | NA |
| PDA and CoA | 1 (0.07%) | 1 (0.12%) | 0 | NA |
| IPS and PDA and aortic stenosis | 1 (0.07%) | 1 (0.12%) | 0 | NA |
ASD, atrial septal defect; CoA, coarctation of the aorta; IPS, infundibular pulmonary stenosis; NA, not applicable; NS, not significant; PBS, pulmonary branch stenosis; PDA, persistent ductus arteriosus.
The majority of VSDs occurred in isolation but in 19.6% of patients they were associated with one other cardiac abnormality, in 2.4% with two other abnormalities, and in only 0.1% with three abnormalities (table 2). Muscular defects were less common in the adult group as would be expected due to spontaneous closure in childhood and adolescence.
Table 2.
Association of VSD with other cardiac abnormalities
| Number of defects | |||
| Paediatric group | Adult group | Overall | |
| Isolated VSD | 657 (78.6%) | 470 (76.8%) | 1127 (77.8%) |
| Perimembranous | 461 (55.1%) | 401 (65.5%) | 862 (59.5%) |
| Muscular | 196 (23.4%) | 69 (11.3%) | 265 (18.3%) |
| One associated abnormality | 150 (17.9%) | 134 (21.9%) | 284 (19.6%) |
| Two associated abnormalities | 27 (3.2%) | 8 (1.3%) | 35 (2.4%) |
| Three associated abnormalities | 2 (0.2%) | 0 | 2 (0.1%) |
| Total | 836 | 612 | 1448 |
Follow up
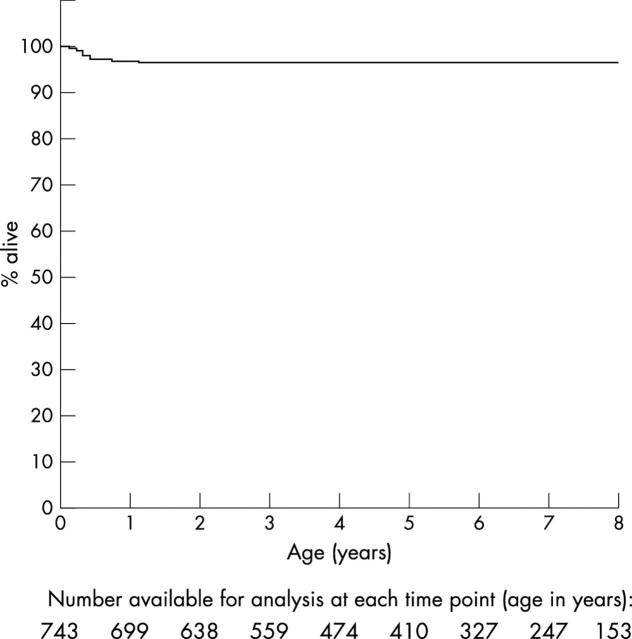
In their first year of life 743 patients were assessed. Figure 2 shows eight years of prospective clinical follow up with 4% cumulative all cause mortality (29 deaths) by the age of 8 years. The majority of deaths occurred during the first year in infants with additional genetic abnormalities as table 3 and table 4 show. No patient developed endocarditis in the follow up cohort. In addition, 16 patients were assessed in the first year of life but were then lost to follow up and have not been included in this analysis. Eleven of these patients had small muscular defects.
Figure 2.
Kaplan-Meier survival curve for new patients followed up since birth.
Table 3.
Deaths in the follow up cohort
| Age at death (years) | Cardiac abnormality | Additional abnormality |
| 0.01 | Isolated VSD | |
| 0.01 | Isolated VSD | Trisomy 18 |
| 0.02 | VSD and secundum ASD | Trisomy 18 |
| 0.03 | Isolated VSD | Trisomy 13 |
| 0.04 | Isolated VSD | Preterm infant, necrotising enterocolitis |
| 0.06 | Isolated VSD | Dysmorphic, Hirschhorn |
| 0.08 | Isolated VSD | Trisomy 13 |
| 0.08 | Isolated VSD | Trisomy 18 |
| 0.09 | Isolated VSD | Trisomy 13 |
| 0.16 | Isolated VSD | Down’s syndrome, duodenal atresia |
| 0.23 | VSD, ASD, PFO, and left PBS | |
| 0.25 | Isolated VSD | 22q11 deletion syndrome |
| 0.27 | VSD and aortic incompetence | Immotile cilia |
| 0.29 | VSD and secundum ASD | |
| 0.30 | Isolated VSD | |
| 0.31 | VSD and IPS | |
| 0.33 | Isolated VSD | |
| 0.37 | Isolated VSD | |
| 0.37 | Isolated VSD | Cleft lip, palate, pseudotrisomy 18 |
| 0.38 | Isolated VSD | Down’s syndrome, preterm |
| 0.41 | Isolated VSD | Preterm infant |
| 0.41 | Isolated VSD | Down’s syndrome (preterm infant) |
| 0.44 | Isolated VSD | |
| 0.49 | VSD and PDA | Multiple congenital abnormalities |
| 0.64 | Isolated VSD | Trisomy 18 |
| 0.74 | Isolated VSD | Holt-Oram syndrome |
| 0.85 | Isolated VSD | Trisomy 18 |
| 0.87 | Isolated VSD |
PFO, patent foramen ovale.
Table 4.
Chromosomal abnormalities in the paediatric group
| Chromosomal abnormality | Number of patients |
| Trisomy 21 | 29 (3.5%) |
| Trisomy 18 | 7 (0.8%) |
| Trisomy 13 | 4 (0.5%) |
| 22q11 deletion | 4 (0.5%) |
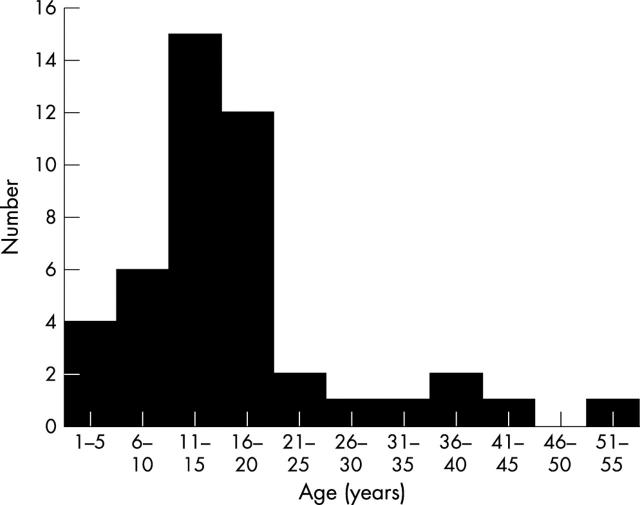
Figure 3 shows an increase in the number of patients with aortic regurgitation with age with a peak between 11 and 15 years of age.
Figure 3.
Age when aortic regurgitation first detected.
Overall 105 patients in the adult group required corrective surgery (including percutaneous closure) although in most cases this was performed in childhood with a mean (SD) age of 4.7 (4.7) years at the time of surgery. During the prospective follow up of the paediatric group 106 patients required corrective surgery (12.6%), and this was performed at a mean (SD) age of 1.4 (2.1) years.
Of the 15 paediatric patients with aortic valve prolapse, moderate or severe regurgitation was detected in five (33%). Of the 46 adult patients with aortic valve prolapse, moderate or severe regurgitation was apparent in five (all of whom underwent surgery with aortic valve replacement in three and cusp resuspension in two) and 19 patients had trivial or mild aortic regurgitation.
DISCUSSION
We have found that about 20% of patients with congenital VSD have additional cardiac abnormalities. Most abnormalities were detected at the initial assessment but aortic prolapse and infundibular pulmonary stenosis may develop subsequently. Such abnormalities may influence management, although the overall prognosis from VSD was excellent in this population and the risk of endocarditis was low.
Secondary aortic regurgitation may result from the venturi effect of high velocity flow beneath an inadequately supported right aortic valve cusp14 or following maldevelopment of the aortic commissure. Continuing clinical follow up is therefore required for patients with VSD beyond adolescence and in our study 82% of cases of secondary aortic regurgitation were detected before the age of 20 years. Extending the follow up of patients to the age of 30 would allow the detection of 89% of patients with secondary aortic regurgitation. The indications for aortic valve surgery in aortic root prolapse are controversial. Recent evidence suggests that the progression from mild to severe incompetence may be rapid and that surgical intervention should be early.15
Echocardiography with colour flow mapping is an effective technique for the detection of VSD16 and in fact is more sensitive than cardiac catheterisation in the detection of multiple septal defects.8 Echocardiography has also been shown to be as sensitive as cardiac catheterisation in the detection of other cardiac abnormalities, such as infundibular pulmonary stenosis.8 Echocardiography continues to develop with advances such as harmonic imaging17 and transducer characteristics18 that allow higher frequency imaging and improved image quality. Colour flow Doppler processing has been the most important advance in the detection of VSD, as flow across the interventricular septum can be easily detected.19 Although other techniques are evolving such as the use of contrast agents,20 three dimensional echocardiography,21,22 and magnetic resonance imaging,23 it seems unlikely that these advances will significantly increase the confirmation rate of clinically suspected VSD or of associated cardiac abnormalities.
This study was not primarily designed to measure long term clinical outcome and all cause mortality was recorded to ensure that cases were not missed because of misclassification of death. The prevalence statistics of associated cardiac abnormalities reflects true clinical practice, as patients were assessed consecutively without selection bias.
Summary
Echocardiography confirms the presence of additional cardiac abnormalities in 22% of patients with congenital VSD. These should be sought during the initial assessment, and the subsequent development of aortic prolapse or infundibular pulmonary stenosis should be monitored during clinical follow up. Follow up to the age of 30 years allows the detection of most but not all cases of secondary aortic regurgitation.
REFERENCES
- 1.Samanek M , Voriskova M. Congenital heart disease among 815,569 children born between 1980 and 1990 and their 15 year survival: a prospective Bohemia survival study. Pediatr Cardiol 1999;20:411–7. [DOI] [PubMed] [Google Scholar]
- 2.Meberg A , Otterstad JE, Froland G, et al. Increasing incidence of ventricular septal defects caused by improved detection rate. Acta Paediatr 1994;83:653–7. [DOI] [PubMed] [Google Scholar]
- 3.Mehta AV, Chidambaram B. Ventricular septal defect in the first year of life. Am J Cardiol 1992;70:364–6. [DOI] [PubMed] [Google Scholar]
- 4.Ramaciotti C , Vetter JM, Bornemeier RA, et al. Prevalence, relation to spontaneous closure, and association of muscular ventricular septal defects with other cardiac defects. Am J Cardiol 1995;75:61–5. [DOI] [PubMed] [Google Scholar]
- 5.Frontera-Izquierdo P , Cabezuelo-Huerta G. Natural and modified history of isolated ventricular septal defect: a 17-year study. Paediatr Cardiol 1992;13:193–7. [DOI] [PubMed] [Google Scholar]
- 6.Kidd L , Driscoll DJ, Gersony WM, et al. Second natural history study of congenital heart defects: results of treatment of patients with ventricular septal defects. Circulation 1993;87 (suppl I) :I38–51. [PubMed] [Google Scholar]
- 7.Magee AG, Fenn L, Vellekoop J, et al. Left ventricular function in adolescents and adults with restrictive ventricular septal defect and moderate left-to-right shunting. Cardiol Young 2000;10:126–9. [DOI] [PubMed] [Google Scholar]
- 8.Magee AG, Boutin C, McCrindle BW, et al. Echocardiography and cardiac catheterisation in the preoperative assessment of ventricular septal defect in infancy. Am Heart J 1998;135:907–13. [DOI] [PubMed] [Google Scholar]
- 9.Gelb BD, O’Laughlin MP, Mullins CE. Prevalence of additional cardiovascular anomalies in patients referred for transcatheter closure of patent ductus arteriosus. J Am Coll Cardiol 1990;16:1680–6. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland GR, Godman MJ, Smallhorn JF, et al. Ventricular septal defects: two dimensional echocardiographic and morphological correlations. Br Heart J 1982;47:316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Fallon WM, Weidman WH, eds. Long-term follow-up of congenital aortic stenosis, pulmonary stenosis, and ventricular septal defect: report from the second joint study of congenital heart defects (NHS-2). Circulation 1993;87 (suppl I) :I1–126. [PubMed] [Google Scholar]
- 12.Craig BG, Smallhorn JF, Burrows P, et al. Cross-sectional echocardiography in the evaluation of aortic valve prolapse associated with ventricular septal defects. Am Heart J 1986;112:800–7. [DOI] [PubMed] [Google Scholar]
- 13.Tohyama K , Satomi G, Momma K. Aortic valve prolapse and aortic regurgitation associated with subpulmonic ventricular septal defect. Am J Cardiol 1997;79:1285–9. [DOI] [PubMed] [Google Scholar]
- 14.Ando M , Takao A. Pathological anatomy of ventricular septal defect associated with aortic valve prolapse and regurgitation. Heart Vessels 1986;2:117–26. [DOI] [PubMed] [Google Scholar]
- 15.Eroglu AG, Oztunc F, Saltik L, et al. Aortic valve prolapse and aortic regurgitation in patients with ventricular septal defect. Pediatr Cardiol 2003;24:36–9. [DOI] [PubMed] [Google Scholar]
- 16.Helmcke F , de Souza A, Nanda NC, et al. Two-dimensional and color Doppler assessment of ventricular septal defect of congenital origin. Am J Cardiol 1989;63:1112–6. [DOI] [PubMed] [Google Scholar]
- 17.Caidahl K , Kazzam E, Lidberg J, et al. New concept in echocardiography: harmonic imaging of tissue without use of contrast agent. Lancet 1998;352:1264–70. [DOI] [PubMed] [Google Scholar]
- 18.Marx GR. Advances in cardiac imaging in congenital heart disease. Curr Opin Pediatr 1995;7:580–6. [DOI] [PubMed] [Google Scholar]
- 19.Ritter SB. Two-dimensional Doppler colour flow mapping in congenital heart disease. Clin Cardiol 1986;9:591–6. [DOI] [PubMed] [Google Scholar]
- 20.Kaul S . New developments in ultrasound systems for contrast echocardiography. Clini Cardiol 1997;20 (10 suppl 1) :I27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Castro S , Yao J, Pandian NG. Three-dimensional echocardiography: clinical relevance and application. Am J Cardiol 1998;81:96G–102G. [DOI] [PubMed] [Google Scholar]
- 22.Dall’Agata A , Cromme-Dijkhuis AH, Meijboom FJ, et al. Three-dimensional echocardiography enhances the assessment of ventricular septal defect. Am J Cardiol 1999;83:1576–9. [DOI] [PubMed] [Google Scholar]
- 23.Didier D , Higgins CB. Identification and localization of ventricular septal defect by gated magnetic resonance imaging. Am J Cardiol 1986;57:1363–8. [DOI] [PubMed] [Google Scholar]