Abstract
Objective: To examine the relation of diabetes and coronary heart disease (CHD; myocardial infarction (MI) or angina) to the incidence of major CHD and stroke events and total mortality.
Methods: Prospective study of 5934 men aged 52–74 years followed up for 10 years. The men were divided into five groups according to their diabetes and CHD status.
Results: During the follow up there were 662 major CHD events, 305 major stroke events, and 1357 deaths from all causes (637 cardiovascular disease (CVD) deaths, 417 CHD deaths). Men with diabetes had significantly increased cardiovascular and total mortality risk compared with non-diabetic men with no CHD but lower risk than men with prior MI only. The adjusted relative risk for CHD deaths was 2.82 (95% confidence interval (CI) 1.85 to 4.28) in men with diabetes only, 2.12 (95% CI 1.53 to 2.93) in men with angina only, 3.91 (95% CI 3.07 to 4.99) in men with MI, and 8.93 (95% CI 6.13 to 12.99) in men with both diabetes and CHD. Case fatality among men with diabetes only was similar to those with prior MI only. CHD and CVD mortality increased with increasing duration of diabetes with risk eventually approaching that of patients with MI without diabetes.
Conclusion: Men with diabetes only have a CVD risk intermediate between men with angina and men with prior MI. Their absolute risk is high and the prognosis for diabetic patients who develop CHD is extremely poor.
Keywords: non-insulin dependent diabetes mellitus, myocardial infarction, cardiovascular disease, mortality
It is well established that non-insulin dependent diabetes is associated with a major increase in risk of coronary heart disease (CHD), cardiovascular disease (CVD), and total mortality. CVD is the leading cause of death of people with diabetes. Previous studies have reported a two- to fourfold increased risk of CVD mortality compared with people who do not have diabetes.1 Additionally, the case fatality rate of CHD is increased among patients with non-insulin dependent diabetes.2 There is evidence suggesting that CVD risk among diabetic patients is equivalent to that associated with prior myocardial infarction (MI) among non-diabetic patients,1–4 which has led to the recommendation in the USA by the National Cholesterol Education Program Adult Treatment Panel III, the American Diabetes Association, and the American Heart Association5–7 that diabetes should be treated as a “CHD equivalent”. However, other large studies have found that a history of MI or a history of CHD (prior MI, angina pectoris, or both) is a stronger predictor of CHD or CVD mortality than diabetes alone.8,9,10 Some studies suggest that the impact on CVD risk of diabetes alone and prior MI without diabetes may depend on sex11 and on duration of diabetes.8,12 However, virtually all these studies have observed that diabetic patients who have CHD have a worse prognosis for survival than patients with CHD without diabetes.1,3,4,8,11,12 We have examined the relation of diabetes and CHD (MI or angina) to the 10 year incidence of major CVD events (CHD, stroke, case fatality, and mortality) caused by CVD and all causes in a population of 5934 British men aged 52–74 years and examined the risk separately among patients with angina and patients with MI. We have also assessed the association between duration of diabetes and outcome.
PATIENTS AND METHODS
The British regional heart study is a large prospective study of CVD among 7735 men aged 40–59 years at initial examination selected from the age–sex registers of one group general practice in each of 24 towns in England, Wales, and Scotland.13 In 1978–80 research nurses administered to each man a standard questionnaire regarding smoking, alcohol, physical activity, and medical history (Q1). A further questionnaire was sent to the men five years later (1983–1985: Q5) to obtain information on medical history and smoking and drinking patterns. In 1992, 12–14 years after initial examination, a similar but more comprehensive questionnaire (Q92) was completed by surviving participants, now aged 52–74 years (average 63 years). In addition to questions on medical diagnoses and current medication, information was obtained on changes in smoking, drinking, and leisure time physical activity. Of the 6528 surviving and available participants, 5934 (91%) completed the Q92 questionnaire.
Cardiovascular risk factors and symptoms
Details of classification methods for smoking status, alcohol intake, physical activity, body mass index (BMI), and social class have been described for this cohort.13–15 A physical activity score was derived for each man and the men were grouped into six broad categories: inactive, occasional, light, moderate, moderately vigorous, and vigorous.14 “Active” refers to patients with light or greater levels of physical activity. From the combined information at initial examination and at Q92 the men were classified at Q92 as those who had never smoked, long term former smokers (men who were non-smokers since Q5), recent former smokers (smokers who gave up smoking between Q5 and Q92), and current smokers. The men were grouped into five alcohol categories on the basis of their estimated weekly intake: none, occasional, light, moderate, and heavy.14 The longest held occupation of each man was recorded at initial examination and the men were grouped into one of six social classes. Non-manual social class comprised social classes I, II, and III non-manual. Manual social class comprised social classes III manual, IV, and V. Those whose longest occupation was in the Armed Forces formed a separate group. BMI (weight (kg)/height (m2)) was calculated for each man based on reported weight at Q92 and on height measured at screening. Overweight is defined as BMI 26.0–29.9 kg/m2 and obesity as BMI ⩾ 30 kg/m2. The men provided information on cardiovascular symptoms such as chest pain on exertion (possible angina), severe chest pain (possible MI), and breathlessness (possible heart failure). Details of classification criteria for these symptoms have been described.15,16
Follow up
All men were followed up from screening to October 2002 for all cause mortality, cardiovascular morbidity, and the development of non-insulin dependent diabetes.17 Information on death was collected through the established tagging procedures provided by the NHS registers. Fatal stroke episodes were those coded on the death certificate to International classification of diseases, 9th revision (ICD-9) codes 430–438. Non-fatal stroke events were those that produced a neurological deficit that was present for more than 24 hours. Fatal CHD events were defined as death with CHD (ICD-9 codes 410–414) as the underlying code. A non-fatal MI was diagnosed according to World Health Organization criteria.18 Evidence regarding CHD and stroke events was obtained by reports from general practitioners, by biennial reviews of the patients’ notes (including hospital and clinic correspondence) through to the end of the study period, and from postal questionnaires to surviving patients at the fifth and 12th to 14th years after initial examination (Q5 and Q92). Cardiovascular deaths were all those with ICD-9 codes 410–439. New cases of non-insulin dependent diabetes were ascertained by postal questionnaires to surviving patients at the fifth and 12th to 14th years after initial examination, by systematic biennial reviews of primary care records, and by review of all death certificates for any reference to diabetes. All events in the period to October 2002 were recorded and follow up has been achieved for 99% of the cohort. Because of the small number of diabetic men and men with MI at initial screening (Q1), we have used data obtained at Q92 (12–14 years later) to assess risk outcome according to diabetes and CHD status. This provides a sufficient number of cases to enable us to compare risk between diabetic patients and patients with MI. The present report is thus concerned only with the men who completed the Q92 questionnaire and with follow up since Q92, giving a follow up period of 10 years for each man.
Men with doctor diagnosed CHD and diabetes
At Q92 the men were asked whether a doctor had ever told them that they had angina or MI (heart attack, coronary thrombosis), stroke, “other heart trouble”, diabetes, and some other disorders. Men with doctor diagnosed CHD were those who recalled a doctor’s diagnosis of CHD (heart attack or angina) at Q92 and those who had had a major non-fatal MI event or angina event before Q92 based on the regular surveillance of general practitioners’ records, including hospital and clinic correspondence (n = 1009). We divided this group into patients with and without MI (based on general practitioners’ records and patient recall). Diabetic patients were men who recalled a doctor’s diagnosis of diabetes at Q92 and men with confirmed diabetes based on general practitioners’ records between initial examination and the completion of Q92 (n = 285). On the basis of their diabetic and CHD status at Q92 the men were divided into five groups:
men without diabetes and without CHD (reference group)
men with a diagnosis of diabetes without CHD
men with a diagnosis of angina only, based on recall of a doctor’s diagnosis of angina on the questionnaire or a general practitioner’s record of angina
men with a diagnosis of MI based on patient recall or a general practitioner’s records
men with a diagnosis of diabetes and a history of CHD (MI or angina) (two thirds of these men had a diagnosis of MI).
Duration of diabetes
Diabetic men without CHD (n = 202) were divided into three groups according to the time of diagnosis of diabetes before Q92:
a history or diagnosis of diabetes before 1980 (> 12 years before Q92)
diagnosis between 1980 and 1985 (7–12 years),
diagnosis between 1985 and 1992 (Q92) (< 7 years).
As an exact year of diagnosis of diabetes was not available for all men, these cut off dates were chosen because they marked the end of the initial examination period (1978–1980) and of the Q5 follow up questionnaire period (1983–1985) when recall of a history of diabetes was obtained.
Case fatality
Case fatality was defined as the proportion of major CHD events in which death occurred during the 28 days after the first event during follow up from Q92, and in which the death certificate recorded CHD (ICD-9 codes 410–414).
Statistical methods
Kaplan-Meier curves were used to construct cumulative 10 year incidence of major CHD events and probability of survival (percentage) for CHD, CVD, and all cause mortality in the five diabetic and CHD status groups. Cox’s proportional hazards model was used to assess the age adjusted and multivariate adjusted relative risk for each category compared with the reference group.19 Adjustments were made for confounding factors measured at Q92. In the adjustment, smoking (never smokers, long term former smokers, recent former smokers, and current smokers), social class (seven groups), BMI (five groups: < 22, 22–23.9, 24–25.9, 26–27.9, and ≥28), physical activity (five groups: inactive/occasional, light, moderate, moderately vigorous/vigorous), and alcohol intake (none, occasional, light, moderate, and heavy) were fitted as categorical variables.
RESULTS
During the 10 years’ follow up of the 5934 men, 1357 died of all causes including 637 CVD deaths (47% of all causes) and 417 CHD deaths; there were 662 major CHD events and 305 major stroke events. Table 1 presents the characteristics at Q92 for the five groups. Men with CHD were generally older than those with diabetes. Diabetic men, with or without CHD, had the highest obesity rates but the prevalence of overweight (26–29.9 kg/m2) did not vary significantly between the five groups. Diabetic men without CHD had higher hypertension rates than non-diabetic men with no CHD, but the prevalence of CVD symptoms (chest pain, severe chest pain, and breathlessness) was only slightly increased. The prevalence of CVD symptoms was very high among men with CHD (with or without diabetes). Men with diabetes and CHD tended to have the worst risk factor profile.
Table 1.
Characteristics obtained from the 1992 questionnaire according to diabetes and coronary heart disease (CHD) status of 5934 British men aged 52–74 years
| Characteristic | No CHD or diabetes | Diabetes only | Angina only | Prior MI | Diabetes and CHD |
| Number | 4723 | 202 | 379 | 547 | 83 |
| Mean age (years) | 62.7 | 63.5 | 65.5 | 64.7 | 65.3 |
| Mean BMI | 26.0 | 27.2 | 26.6 | 26.5 | 27.1 |
| Obese | 9.8% | 21.8% | 12.7% | 15.5% | 22.5% |
| Overweight | 35.2% | 36.1% | 40.1% | 34.0% | 32.5% |
| Never smokers | 28.4% | 24.0% | 17.5% | 16.2% | 15.7% |
| Current smokers | 20.9% | 12.9% | 17.2% | 16.5% | 19.3% |
| Physically active | 45.2% | 36.7% | 31.7% | 33.8% | 20.6% |
| Manual labourers | 54.9% | 55.9% | 62.9% | 59.7% | 65.4% |
| Non-drinkers | 15.8% | 22.1% | 23.7% | 22.5% | 34.6% |
| Recall of hypertension | 22.0% | 36.1% | 41.2% | 37.4% | 37.4% |
| Recall of stroke | 2.6% | 5.9% | 6.6% | 8.4% | 14.5% |
| Chest pain on exertion | 5.1% | 6.4% | 56.7% | 52.3% | 53.0% |
| Severe chest pain (possible MI) | 3.1% | 3.5% | 21.9% | 51.7% | 42.2% |
| Breathlessness | 16.6% | 19.3% | 49.9% | 61.4% | 59.0% |
BMI, body mass index; MI, myocardial infarction.
The cumulative event rate among diabetic men without CHD (19.0%) was similar to the rate among men with a doctor diagnosis of angina only (19.9%). Men with diagnosed MI had a 29% event rate and this increased to 50.2% among men with both diabetes and CHD compared with 8.6% in men with no diabetes or CHD. Figure 1 shows the Kaplan-Meier estimates of the probability of survival for CHD, CVD, and all cause mortality stratified by history of diabetes and CHD. Men with both diabetes and CHD had by far the worst prognosis. These men had a less than 50% survival probability for CVD death compared with 71% for those with MI, 82% for men with angina only, 81% for men with diabetes only, and 92% for men with no diabetes or CHD. Considering all cause mortality, only a third of the men with diabetes and CHD were alive after 10 years.
Figure 1.
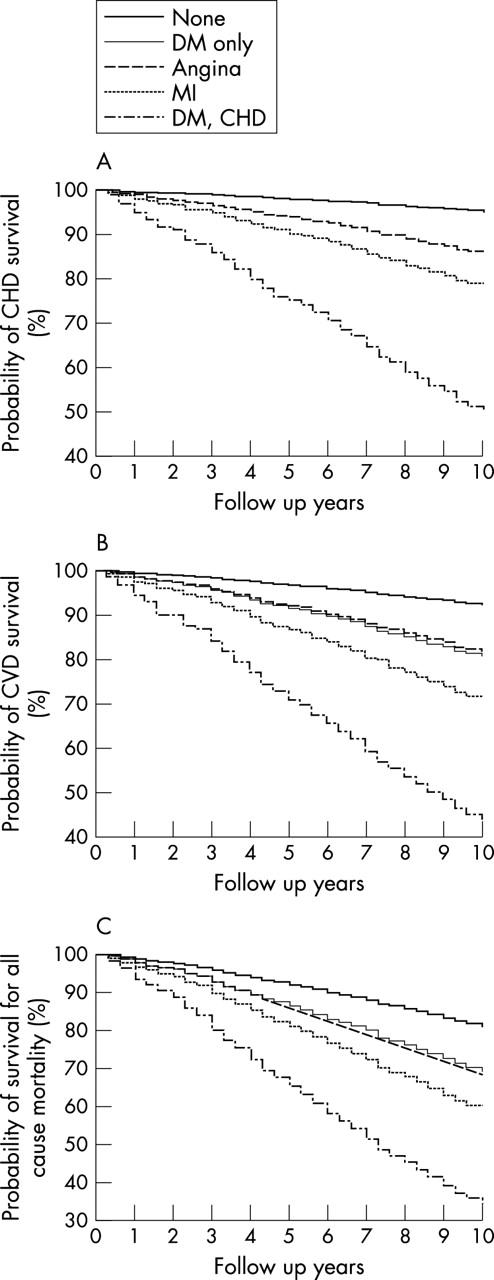
Kaplan-Meier estimates of probability of survival (%) for (A) coronary heart disease (CHD), (B) cardiovascular disease (CVD), and (C) all cause mortality stratified by history of diabetes (DM) and CHD. Survival probabilities were virtually identical for the angina group and the DM only group. MI, myocardial infarction.
Table 2 shows the rates/1000 person-years and adjusted relative risks for major CHD events, CHD deaths, major stroke events, and deaths from CVD and all causes for the five groups. The diabetes only group had significantly higher adjusted risk of major CHD events, CHD deaths, stroke events, and CVD death than non-diabetic men with no CHD, and the adjusted relative risks were higher than for those with angina only. Men with MI had a higher risk of cardiovascular outcomes (with the exception of stroke) than diabetic men without CHD, but risk was by far the highest in men with diabetes and CHD. These men had nearly a ninefold increase in risk of CHD death compared with non-diabetic men with no CHD, whereas this was nearly three- to fourfold in men with diabetes or CHD. Although those with diabetes only were less likely to have a major CHD event than men with prior MI, their case fatality was similar to that in men with prior MI. Men with diabetes and CHD were very unlikely to survive a subsequent MI, with case fatality rates of nearly 90%. Total mortality was similar in the diabetes and the CHD groups but was by far the highest among men with both conditions. In men with a history of diabetes or CHD (angina or MI) over half of all deaths were due to CVD causes, and in men with diabetes and CHD the vast majority died of CVD causes (78%). Overall, men with diabetes only had CHD and CVD mortality and total mortality outcomes intermediate between non-diabetic men with angina and men with prior MI.
Table 2.
Rates/1000 person-years (p-y) and adjusted relative risk (RR) for major CHD and stroke events and CHD deaths, and for CVD deaths and all cause mortality according to doctor diagnosed diabetes and prior CHD status of men aged 52–74 years
| No CHD or diabetes | Diabetes only | Doctor diagnosed angina only | Doctor diagnosed MI | Diabetes and CHD | |
| Number | −4723 | −202 | −379 | −547 | −83 |
| Major CHD events | |||||
| Number | 382 | 36 | 68 | 140 | 36 |
| Rate/1000 p-y | 9.0 | 21.0 | 22.0 | 33.9 | 69.0 |
| Adjusted† RR | 1.00 | 2.09 (1.48 to 2.96) | 1.89 (1.45 to 2.46) | 3.16 (2.59 to 3.86) | 5.31 (3.73 to 7.57) |
| All CHD deaths | |||||
| Number | 207 | 26 | 47 | 102 | 35 |
| Rate/1000 p-y | 4.8 | 14.9 | 14.7 | 23.4 | 64.8 |
| Adjusted† RR | 1.00 | 2.82 (1.85 to 4.28) | 2.12 (1.53 to 2.93) | 3.91 (3.07 to 4.99) | 8.93 (6.13 to 12.99) |
| Case fatality | 51.6% | 69.4% | 63.2% | 69.3% | 88.9% |
| Adjusted† RR | 1.00 | 2.12 (0.94 to 4.77) | 1.10 (0.61 to 1.98) | 2.09 (1.33 to 3.29) | 8.47 (2.76 to 26.0) |
| Major stroke events* | |||||
| Number | 208 | 20 | 22 | 47 | 8 |
| Rate/1000 p-y | 4.9 | 11.8 | 7.1 | 11.3 | 16.4 |
| Adjusted† RR | 1.00 | 2.41 (1.52 to 3.03) | 1.08 (0.68 to 1.70) | 2.01 (1.46 to 2.78) | 2.60 (1.27 to 5.30) |
| CVD deaths | |||||
| Number | 349 | 37 | 63 | 146 | 42 |
| Rate/1000 p-y | 8.0 | 21.2 | 19.7 | 33.5 | 77.8 |
| Adjusted† RR | 1.00 | 2.42 (1.71 to 3.42) | 1.68 (1.28 to 2.21) | 3.28 (2.69 to 4.00) | 6.27 (4.49 to 8.75) |
| Total deaths | |||||
| Number | 900 | 63 | 118 | 222 | 54 |
| Rate/1000 p-y | 20.8 | 36.0 | 36.8 | 50.9 | 100.0 |
| Adjusted† RR | 1.00 | 1.72 (1.33 to 2.24) | 1.31 (1.08 to 1.59) | 2.04 (1.76 to 2.38) | 3.27 (2.47 to 4.34) |
| Deaths due to CVD | 39% | 58.7% | 53.4% | 65.8% | 77.8% |
*Excluding 96 men with pre-existing confirmed doctor diagnosis of stroke.
†Adjusted for age, smoking, social class, BMI, physical activity, and alcohol intake.
We also directly compared the risk of the major outcomes between men with CHD and men with diabetes (table 3). Men with angina only had slightly lower (non-significant) risk of CHD and CVD outcome than men with diabetes only. Men with prior MI had significantly higher risk of CHD events than men with diabetes without CHD and higher risk of CHD and CVD deaths, but the numbers were small and the differences were of marginal significance (p = 0.08 and p = 0.06, respectively). Diabetes with CHD increased the risk threefold for CHD events and deaths and CVD deaths compared with diabetes without CHD.
Table 3.
Adjusted RR of major CHD and stroke events, CHD, CVD deaths and total mortality compared with diabetic men
| Diabetes only | Doctor diagnosed angina only | Doctor diagnosed MI | Diabetes and CHD | |
| Major CHD events | ||||
| Adjusted† RR | 1.00 | 0.92 (0.61 to 1.39) | 1.59 (1.09 to 2.31) | 2.76 (1.71 to 4.45) |
| CHD deaths | ||||
| Adjusted† RR | 1.00 | 0.79 (0.48 to 1.30) | 1.47 (0.94 to 2.29) | 3.56 (2.09 to 6.05) |
| Major stroke events* | ||||
| Adjusted† RR | 1.00 | 0.48 (0.20 to 0.90) | 0.87 (0.51 to 1.49) | 1.15 (0.50 to 2.69) |
| CVD deaths | ||||
| Adjusted† RR | 1.00 | 0.73 (0.48 to 1.10) | 1.42 (0.98 to 2.05) | 2.86 (1.80 to 4.53) |
| Total death | ||||
| Adjusted† RR | 1.00 | 0.81 (0.59 to 1.11) | 1.25 (0.94 to 1.67) | 2.13 (1.43 to 3.11) |
*Excluding 96 men with pre-existing confirmed doctor diagnosed stroke.
†Adjusted for age, smoking, social class, BMI, physical activity, and alcohol intake.
We also examined the risk of major CVD outcomes by duration of diabetes among men with diabetes without CHD (table 4). Risk of CHD events, CHD deaths, and CVD deaths increased with increasing duration of diabetes. Risk among men with diabetes diagnosed > 12 years before screening approached the risk among men with MI (table 2).
Table 4.
Duration of diabetes and adjusted RR of major CHD events and death from CHD, CVD, and all causes in men with no diagnosis of CHD
| No diabetes | Duration of diabetes | p Value for trend | |||
| <7 years | 7–12 years | >12 years | |||
| Number | 4723 | 119 | 36 | 47 | |
| Major CHD events | |||||
| Number | 382 | 16 | 8 | 12 | |
| Adjusted† RR | 1.00 | 1.67 (1.01 to 2.75) | 2.65 (1.31 to 5.35) | 2.67 (1.46 to 4.88) | <0.0001 |
| CHD deaths | |||||
| Number | 207 | 11 | 5 | 10 | |
| Adjusted† RR | 1.00 | 2.16 (1.17 to 3.97) | 3.23 (1.33 to 7.88) | 4.04 (2.86 to 7.93) | <0.0001 |
| CVD deaths | |||||
| Number | 349 | 19 | 6 | 12 | |
| Adjusted† RR | 1.00 | 2.24 (1.41 to 3.56) | 2.23 (0.99 to 5.01) | 3.01 (1.64 to 5.51) | <0.0001 |
| Total deaths | |||||
| Number | 900 | 37 | 9 | 17 | |
| Adjusted† RR | 1.00 | 1.74 (1.24 to 2.43) | 1.39 (0.72 to 2.68) | 1.84 (1.12 to 3.03) | 0.0002 |
†Adjusted for age, smoking, social class, BMI, physical activity and alcohol intake.
DISCUSSION
In this prospective study of British men aged 52–74 years, diabetes was associated with a significant increase in risk of major cardiovascular events compared with non-diabetic men with no history of CHD. Men with diabetes only had a nearly threefold increase in risk of death from CHD after adjustment for age and confounders, which was higher than the risk in men with angina only but lower than the risk seen in men with prior MI. Not only were patients with diabetes at increased risk but survival in the event of a first heart attack was much less likely. Increased case fatality in diabetic patients compared with non-diabetic patients is well documented2,20 and this study suggests that in this age group, fatality rates are similar to the rates in men with MI despite lower incidence of major CHD events. The high case fatality among diabetic patients may relate to disturbances in haemostasis, impaired fibrinolysis, hypercoagulability, and increased platelet aggregation often present in diabetes.21 These mechanisms may have a role in enhancing and accelerating thrombotic formation in patients with diabetes.21 They may also contribute to the exceptionally poor prognosis for recurrent CHD events and CHD death among with both diabetes and CHD who had almost a ninefold increase in risk of death.
Previous studies
Results of previous studies in high risk populations comparing risk between diabetic patients and patients with prior MI have differed possibly due to the variety of definitions used for inclusion in CHD and CVD categories. Although several studies have shown that the magnitude of risk of CHD events among patients with diabetes is similar to that for recurrent events among non-diabetic patients with established CHD, others have shown lower risk in diabetic patients. In a Finnish population there was a similar risk of CHD mortality among diabetic patients without a prior MI and non-diabetic patients with prior MI.1 An American study also found that diabetes and a previous MI had similar cardiovascular and total mortality risks.3 A multinational study (OASIS (organization to assess strategies for ischemic syndromes)) found that diabetes and previous vascular disease (unstable angina or non-Q wave MI) conferred similar risk of CHD mortality.4 In contrast to these studies, two large American cohorts and two European studies (Scottish and Dutch) observed a higher risk of CHD death associated with prior MI or history of CHD (MI, angina, or both) than with diabetes only,8,9,10,11 although in the Dutch study this was seen in men only.11 In women, diabetes carried a CVD risk similar only to prior CVD without diabetes. This suggests that the impact of diabetes and prior MI on CVD risk may depend on sex. The magnitude of increased risk for CVD or CHD deaths in these studies ranged from about 1.9-fold in a study of American male physicians10 to almost threefold in the Scottish study9 compared with about a 1.5-fold increase in the present study.
Duration of diabetes
We have observed CVD risk to increase with increasing duration of diabetes. Although earlier studies have not observed an association between duration of diabetes and mortality risk,22,23 our findings are consistent with results from two large American cohorts. The health professionals follow up study and nurses’ health study in the United States both found that risk of CHD increased with increasing duration of diabetes.8,12 Furthermore, our results also indicated a level of risk in diabetics approaching that in MI patients without diabetes with increasing duration of diabetes similar to that observed in the nurses’ health study, where women with more than 15 years’ duration of diabetes had risk similar to that of women with a history of CHD (prior MI) and no diabetes12 Whether patients with diabetes alone have a cardiovascular risk equivalent to patients with prior MI may depend on the duration of diabetes.
Potential limitations of the study
Although ECG measurements were not available to identify silent MI, which may be high in patients with diabetes, evidence of possible undiagnosed CHD as assessed by the World Health Organization questionnaire (for example, chest pain on exertion, severe chest pain, and breathlessness) was not greater in diabetic patients with no diagnosed CHD than in non-diabetic patients with no CHD. This suggests that the findings are not biased by the presence of silent MI. Although some misclassification may exist between insulin dependent and non-insulin diabetes, the overwhelming majority of our patient had non-insulin dependent diabetes. Only 17 men who reported diabetes before screening were taking insulin at that time and might have been misclassified as having non-insulin dependent diabetes.
Diabetes as a CHD risk equivalent
The increased risk associated with diabetes appears to be independent of the conventional metabolic risk factors such as obesity, hypertension, and dyslipidaemia.21,24 Although it is still debated whether diabetic patients have a risk of CVD events identical to that of patients with prior MI, the absolute risk in these patients is high. Most of these patients will have had the disease for many years before the diagnosis is established, and a high proportion will have risk factors for CVD responsive to changes in lifestyle (diet, body weight, physical activity, smoking) or to medication. There is already considerable evidence that aspirin,25 statins,26,27 and angiotensin converting enzyme inhibitors28 can greatly diminish the risk of cardiovascular death in patients with diabetes.29 The recent American Diabetes Association and National Cholesterol Education Program guidelines placed particular emphasis on the need for aggressive risk factor reduction for among patients with diabetes and recommend aggressive low density lipoprotein lowering treatment for diabetic patients.5,6 Several secondary prevention trials have shown that patients with diabetes receive at least as much benefit from statin treatment as non-diabetic patients with CHD.30–33 Results from the recent heart protection study further show the benefits of statin treatment in diabetics without CHD.26 While this aggressive approach seems reasonable and evidence based in primary prevention —that is, in diabetic patients without established CVD—it appears to be mandatory in diabetics with established CHD (angina or prior MI) for whom the prognosis is extremely grave.34
Conclusion
Men with diabetes have a cardiovascular and total mortality risk intermediate between the risk in men with angina and men with prior MI. The high death and case fatality rates among diabetic patients with CHD seen in this and other studies emphasises the importance of early and aggressive risk factor intervention in preventing heart attacks and stroke in diabetic patients. Our data support the recommendation that diabetes without CHD be treated as a CHD risk equivalent.5–7
Acknowledgments
The British regional heart study is a British Heart Foundation Research Group and receives support from the Department of Health (England). The views expressed in this publication are those of the authors and not necessarily those of the Department of Health (England).
Abbreviations
BMI, body mass index
CHD, coronary heart disease
CI, confidence interval
CVD, cardiovascular disease
ICD-9, International classification of diseases, 9th revision
MI, myocardial infarction
OASIS, organization to assess strategies for ischemic syndromes
Q1, Q5, Q92, questionnaires administered in the first year, fifth year, and 1992
REFERENCES
- 1.Haffner SM, Lehto S, Ronnema T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34. [DOI] [PubMed] [Google Scholar]
- 2.Mittinen H, Lehto S, Salomma VV, et al. Impact of diabetes on mortality after the first myocardial infarction. Diabetes Care 1998;21:69–75. [DOI] [PubMed] [Google Scholar]
- 3.Mukamal KJ, Maclure M, Nesto RW, et al. Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care 2001;24:1422–7. [DOI] [PubMed] [Google Scholar]
- 4.Malmberg K, Yusuf S, Gerstein H, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: result of the OASIS (organization to assess strategies for ischemic syndromes) registry. Circulation 2000;102:1014–9. [DOI] [PubMed] [Google Scholar]
- 5.Anon. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;385:2486–97. [DOI] [PubMed] [Google Scholar]
- 6.Anon. American Diabetes Association: management of dyslipidemia in adults with diabetes. Diabetes Care 2000;23 (suppl I) :S57–60. [PubMed] [Google Scholar]
- 7.AHA conference proceedings. Prevention conference V: beyond secondary prevention, identifying the high risk patient for primary prevention: executive summary. American Heart Association. Circulation 2000;101:111–6. [DOI] [PubMed] [Google Scholar]
- 8.Cho E, Rimm EB, Stampfer MJ, et al. The impact of diabetes mellitus and prior myocardial infarction on mortality from all causes and from coronary heart disease in men. J Am Coll Cardiol 2002;40:954–60. [DOI] [PubMed] [Google Scholar]
- 9.Evans JMM, Wang J, Morris AD. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: cross sectional and cohort studies. BMJ 2002;324:939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotufo PA, Gaziano M, Chae CU, et al. Diabetes and all cause and coronary heart disease mortality among US male physicians. Arch Intern Med 2001;161:242–7. [DOI] [PubMed] [Google Scholar]
- 11.Becker A, Bos G, de Vegt F, et al. Cardiovascular events in type 2 diabetes: comparison with nondiabetic individuals without and with prior cardiovascular disease: 10-year follow-up of the Hoorn Study. Eur Heart J 2003;24:1406–13. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years follow-up. Arch Intern Med 2001;161:1717–23. [DOI] [PubMed] [Google Scholar]
- 13.Shaper AG, Pocock SJ, Walker M, et al. British regional heart study: cardiovascular risk factors in middle-aged men in 24 towns. BMJ 1981;283:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality: explaining the U-shaped curve. Lancet 1988;ii:1268–73. [DOI] [PubMed] [Google Scholar]
- 15.Shaper AG, Wannamethee G, Weatherall R. Physical activity and ischaemic heart disease in middle-aged men. Br Heart J 1991;66:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook DG, Shaper AG, Macfarlane PW. Using the WHO (Rose) angina questionnaire in cardiovascular epidemiological studies. Int J Epidemiol 1989;18:607–13. [DOI] [PubMed] [Google Scholar]
- 17.Walker M, Shaper AG, Lennon L, et al. Twenty years follow-up of a cohort based in general practices in 24 British towns. J Public Health Med 2000;22:479–85. [DOI] [PubMed] [Google Scholar]
- 18.Rose G, Blackburn H, Gillum RF, et al. Cardiovascular survey methods, 2nd edn. Geneva: World Health Organization, 1982.
- 19.Cox DR. Regression models and life tables (with discussion). J R Stat Soc (B) 1972;34:187–220. [Google Scholar]
- 20.Abbott RD, Donahue RP, Kannel WB, et al. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham study. JAMA 1988;260:3456–60. [PubMed] [Google Scholar]
- 21.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology and management. JAMA 2002;287:2570–81. [DOI] [PubMed] [Google Scholar]
- 22.Jarrett RJ, Shipley MJ. Type 2 diabetes mellitus and cardiovascular disease: putative association via common antecedents: further evidence from the Whitehall study. Diabetologia 1988;31:737–40. [DOI] [PubMed] [Google Scholar]
- 23.Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London cohort of the WHO multinational study of vascular disease in diabetics) II: associated risk factors. Diabetologia 1990;33:542–8. [DOI] [PubMed] [Google Scholar]
- 24.Winocour PH, Fisher M. Prediction of cardiovascular risk in people with diabetes. Diabet Med 2003;20:515–27. [DOI] [PubMed] [Google Scholar]
- 25.Anon. Aspirin therapy in diabetes. American Diabetes Association. Diabetes Care 2003;26:S87–8. [DOI] [PubMed] [Google Scholar]
- 26.Anon. MRC/BHF protection study of cholesterol lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Heart Protection Study Collaborative Group. Lancet 2003;361:2005–16. [DOI] [PubMed] [Google Scholar]
- 27.Anon. Management of dyslipidemia in adults with diabetes. American Diabetes Association. Diabetes Care 2003;26:S83–6. [DOI] [PubMed] [Google Scholar]
- 28.Anon. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355:253–9. [PubMed] [Google Scholar]
- 29.Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93. [DOI] [PubMed] [Google Scholar]
- 30.Pyorala K, Pederson TR, Kjekshus J, et al. The Scandinavian Simvastatin Survival Study (4S) Group: Diabetes Care 1997;20:614–20. [DOI] [PubMed] [Google Scholar]
- 31.Haffner SM, Alexander CM, Cook TJ, et al. for the Scandinavian Simvastatin Survival Study Group. Reduced coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses from the Scandinavian Simvastatin Survival Group. Arch Intern Med 1999;159:2661–7. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg RB, Mellies MJ, Sacks FM, et al. for the CARE investigators. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. Circulation 1998;98:2513–9. [DOI] [PubMed] [Google Scholar]
- 33.Anon. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998;339:1349–57. [DOI] [PubMed] [Google Scholar]
- 34.Dhatariya K. Type 2 diabetes is cardiovascular disease. J R Soc Med 2003;96:371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


