Defibrillation has been used to treat disturbances of the cardiac rhythm since the late 1800s.1w1 In order to appreciate the mechanisms of defibrillation, one must also understand the basic electrophysiological principles of fibrillation.
ELECTROPHYSIOLOGICAL BASIS OF FIBRILLATION
Atrial and ventricular fibrillation are both based upon a re-entrant mechanism, a phenomenon that was first recognised almost 100 years ago and initially described by Mines and Garrey.w1 Mines also suggested that conduction block was necessary to facilitate re-entry.w1 It was not until the 1950s that Moe and colleagues advanced the circus movement theory of re-entry when they suggested the multiple wavelet hypothesis of atrial fibrillation (AF).2 This proposed that AF occurred in the presence of inhomogeneous atrial tissue.2 It was postulated that multiple wavelets propagated randomly through the atria. These waves of excitation were presumed capable of spreading through the atrial myocardium at a time when some of its components had recovered while others remained partially or fully refractory as a result of a preceding activation.2 Thus AF could be maintained as a turbulent arrhythmia in a stable state for long periods of time. By 1985, after the development of high resolution electrode mapping systems, Allessie and colleagues provided evidence of multiple propagating wavelets that created turbulent atrial activity in the canine heart.2 Subsequent experiments have proven Moe’s idea that multiple wavelets distributed randomly throughout the atria gave rise to the chaotic activation patterns observed on the ECG.2
More recent studies have further expanded on some of the experimental findings of Allessie and colleagues, suggesting that some forms of AF may actually be the result of high frequency activation by a single re-entrant source in some patients.2 The drivers of AF may be relatively stationary “rotors” of electrical activity that are anchored to an unexcited (anatomical) core in the posterior left atrium.2 The atrial anatomy may facilitate this re-entry process by providing areas of conduction block (for example, pulmonary vein ostia). It is also increasingly recognised that ectopic foci originating from the pulmonary veins may be responsible for initiation of AF in patients, and that electrical isolation of these foci may provide long term correction of AF in some patients.3 Thus, AF is based upon re-entry. Multiple wavelets of electrical activity propagate randomly in the atrial tissue producing a chaotic but continuous activation of the atrial tissue. Defibrillation (or direct current cardioversion) in this setting aims to bring an abrupt halt to this process and restore sinus rhythm.
MECHANISMS OF ATRIAL DEFIBRILLATION
There are only limited data that examine the interaction between an electrical shock and atrial tissue.4w2 w3 Research into the mechanisms of defibrillation has occurred almost exclusively in the setting of ventricular fibrillation. While there may be distinct differences between the precise mechanisms of atrial and ventricular defibrillation, none has been described. There are several theories of the mechanisms of defibrillation that are derived by ventricular defibrillation.
It is thought that shocks defibrillate by altering the potential difference across the cell membrane (that is, the transmembrane potential).5 However, the situation in the heart is extremely complex with the intracellular space and extracellular space both possessing different electrical properties than the cell membrane that divides them.5 Unfortunately, there is no current consensus on how the extracellular potential gradient relates to the transmembrane potential gradient. Thus, the exact mechanism of electrical defibrillation remains incompletely understood.
The critical mass theory proposes that defibrillation can be successfully achieved by the depolarisation of a sufficient or “critical” mass of tissue. Zipes and colleagues demonstrated that chemical depolarisation of a critical mass of ventricular myocardium (∼75%), using selective infusion of potassium chloride into the left coronary artery, would successfully abolish ventricular fibrillation.5,6 Therefore, if a sufficient voltage potential gradient could be generated by a shock to halt the fibrillation activation fronts in the myocardium, fibrillation could be abolished.5
When an electrical field with strength of 5 V/cm or less is introduced to ventricular myocardium, it exhibits an all or nothing response.5 Therefore, recovered myocardium produces a new action potential, while the stimulus has no effect on even partially refractory cells.5 Above 5 V/cm a graded response can be produced in refractory cells.5 The result of this graded response is a prolongation of both refractoriness and the action potential, although of shorter duration than the production of a new action potential.5 These observations form the basis of the “extension of refractoriness hypothesis”. This hypothesis states “the defibrillating shock interacts primarily with cells during their refractory period and prolongs refractoriness in most of the myocardium so that fibrillation wavefronts cannot propagate and fibrillation ceases”.w4
A lower limit of vulnerability also exists for ventricular myocardium.7 This is the minimum voltage required by an electrical stimulus to induce fibrillation during the vulnerable period. It was noted in the 1960s that there was an upper limit to the strengths of shocks delivered during the vulnerable period that induce ventricular fibrillation.7 It was also observed that the strengths of these shocks at “the upper limit of vulnerability” were approximately equivalent to the shocks at defibrillation threshold.7 The upper limit of vulnerability hypothesis for defibrillation states “to defibrillate, a shock not only must halt the activation fronts of fibrillation, but it also must not reinitiate fibrillation by the same mechanism that a shock of the same strength during the vulnerable period of sinus or paced rhythm initiates fibrillation”.7
These theories are supported by the Fundamental Law of Electrostimulation, which was adapted by Irnich and applied to ventricular defibrillation.w5 If the concept of nearfield and farfield rheobase (lowest current strength which successfully stimulates myocardium) are introduced, then attempted defibrillation with shocks of too low voltage may be effective close to the electrode (nearfield) but fail to stimulate cardiac tissue at a remote site (farfield).w6 In order to gain a more accurate picture of the cellular response to electrical stimuli, optical mapping techniques were developed. This involves the use of potentiometric fluorescent dyes that bind to cell membranes in proportion to the polarisation of the membrane caused by a shock.7 Using this technique, Kwaku and Dillon reported that activation fronts in the ventricles arose from the border of shock-depolarised areas and that these fronts propagated unidirectionally away from these regions.7,8 They also found that increasing refractoriness decreased the likelihood of a reactivation wavefront, and concluded that for successful defibrillation a shock must depolarise myocardial tissue, even during its refractory period.8
Transmembrane potentials have been recorded from the atria during AF and after biphasic shocks.4 The shocks have been shown to produce four types of responses: immediate cessation of epicardial activity, single post-shock activation, organised activation for 0.8–1.5 seconds followed by termination, and organised activity followed by degeneration back into AF.4 These experiments led the authors to conclude, “These results are consistent with both the critical mass hypothesis and the upper limit of vulnerability hypothesis”.4 Therefore, given the similarity between atrial and ventricular myocytes, it seems reasonable to assume that the effects of an electric shock are similar in both chambers.
DEFIBRILLATION WAVEFORMS
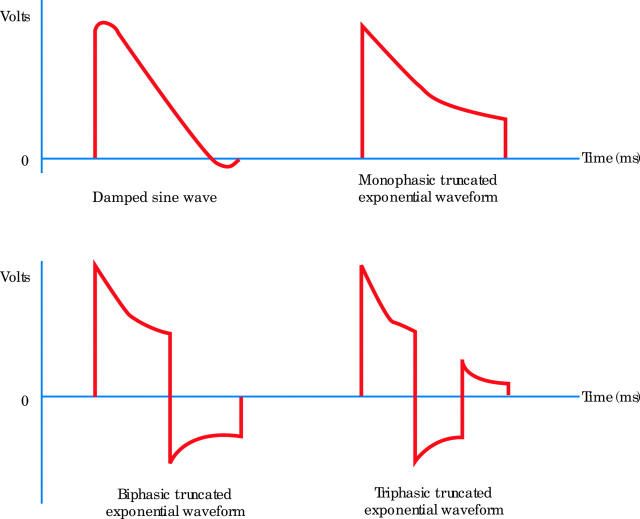
Early devices employed both alternating and direct current for defibrillation. However, by the 1960s it was apparent that alternating current was more detrimental to the heart and direct current has been used in defibrillators since.w7 There are a variety of different waveforms in use today (fig 1).
Figure 1.
Examples of some monophasic, biphasic, and triphasic waveforms (3–20 ms in duration).
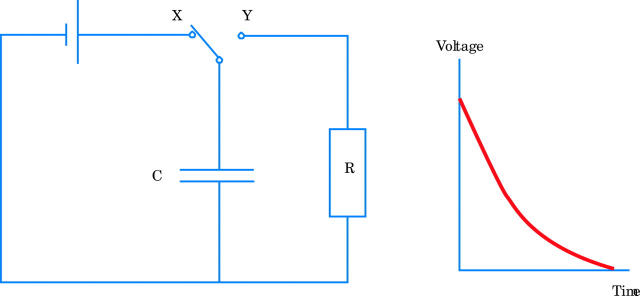
The critically damped monophasic waveforms have been the mainstay of external defibrillation for over three decades. However, these are produced with inductors used in conjunction with capacitors.w7 Inductors are electrical components that are too large for implantable devices. Interest in more recent years has therefore focused on straightforward capacitor discharges, as capacitors were suitable for use in implantable defibrillators. Consider fig 2. When the switch is in position X, the capacitor C will charge until the potential difference across it is equivalent to the potential difference across the battery. When the switch is moved to position Y, exponential discharge occurs through the resistor R (as shown in the graph of voltage versus time).
Figure 2.
A capacitor–resistor circuit and a graph showing exponential capacitor discharge.
The rate of capacitor discharge is described by the equation Q = Q0 e −t/CR. Q represents the charge on the capacitor at a given time, Q0 is the charge at time zero, t is time and e is the exponential function. The time constant of the circuit (amount of time required for charge to reduce to 37% of its initial value) is therefore proportional to CR (the capacitance value and the resistance through which the discharge occurs). Adjusting capacitance allows the slope or “tilt” of the resultant waveform to be controlled. Duration can be truncated at the desired point. Switching the polarity of the circuit during the discharge produces biphasic and triphasic waveforms. The energy produced by a waveform is calculated from the product of voltage and current (power) multiplied by time.
There has been a gradual switch to biphasic waveforms in more recent years due to improved performance in various clinical trials over their monophasic counterparts. It is generally agreed that the first phase of a biphasic waveform should have a positive polarity and duration of equal to or greater than the second phase of the waveform. The actual electrode polarity (placing the anode in the apical or alternative pad position) does not influence the efficacy of biphasic waveforms. It has been shown that biphasic shocks produced less dispersion of refractory period extension and suggested that a more homogeneous dispersion of refractory period extension was a more important factor in explaining why biphasic shocks are more efficacious in ventricular defibrillation.9 In effect a biphasic shock produces less heterogeneous repolarisation and is therefore less likely to re-induce fibrillation.
PRACTICE OF ATRIAL DEFIBRILLATION (DIRECT CURRENT CARDIOVERSION)
Waveforms
Several studies of epicardial and endocardial atrial defibrillation have demonstrated biphasic waveforms to be superior to their monophasic counterparts of equivalent overall duration. The biphasic waveforms offer superior efficacy in terms of peak voltage, current and total energy delivered. This has been shown in animal modelsw8 of AF as well as in patients.w9 w10
The development of devices that deliver impedance compensated biphasic (ICB) waveforms for use in cardiac arrest has stimulated interest in their use for direct current cardioversion. The evidence to date shows that the biphasic devices offer successful cardioversion rates comparable to or superior to standard monophasic defibrillators, but at significantly lower energies (table 1).10,11w11 w12 Some physicians may argue that biphasic devices do not offer a clear advantage over monophasic defibrillators. Arguments include: there is no large increase in overall success of the procedure (when both protocols are completed to the highest energy); there does not appear to be any functional advantage for the patient from the use of biphasic waveforms; biphasic devices have not been shown to influence the duration of sinus rhythm; and exposure to high cumulative energies does not lead to troponin release.w13
Table 1.
Success of monophasic (MP) and biphasic (BP) waveforms at cumulative energy levels in cardioversion of atrial fibrillation (AF)
| Study | MP energy selection protocol (J) | Overall MP success (%) | BP device | BP energy selection protocol (J) | Overall BP success (%) |
| Mittal et al10 | 100, 200, 300, 360 | 79 | Zoll PD-2100 | 70, 120, 150, 170 | 94 |
| Page et al11 | 100, 150, 200, 360 | 85 | Heartstream XL | 100, 150, 200, 200 | 91 |
| Scholten et alw11 | 200, 360 | 90 | Zoll M-Series | 120, 200 | 90 |
| Marinsek et alw12 | 100, 200, 300, 360 | 90 | Heartstream XL | 70, 100, 150, 200 | 88 |
However, skin burns are less common with biphasic waveforms11 with the likelihood of a skin burn increasing proportionally with the total energy delivered for both monophasic and biphasic waveforms.11w14 Biphasic cardioversion is also associated with less skeletal muscle damage.w12 In addition, fewer shocks are needed with a biphasic device,11 potentially leading to shorter cardioversion procedures. Biphasic waveforms have been shown to be efficacious for patients with previously failed attempted cardioversion with monophasic waveforms.w15 Finally, given the clear advantages of impedance compensated biphasic waveforms, monophasic devices in hospital are likely to be phased out and replaced by their biphasic counterparts. This will result in an inevitable shift in practice toward biphasic direct current cardioversion of atrial arrhythmias.
Electrode positions
Historically, anteroposterior (AP) configurations have been considered to provide a superior “shock vector” through the atria compared with the antero-apical (AA) configuration.w16 w17 However, the actual path taken by current through human tissue is complex, with a small percentage (∼4%) of current delivered by the transthoracic route reaching the heart in the AA configuration.w18 The route taken by current during AP defibrillation has not been described. Two studies have suggested that an AP electrode configuration is superior to an AA electrode configuration when monophasic waveforms are used for direct current cardioversion of AF.12,13 However, neither study reported whether there was a difference in transthoracic impedance for the configurations studied. Transthoracic impedance (TTI) has been shown to be an important determinant of atrialw19 and ventricular defibrillationw20 and is now widely accepted as an important predictor of success.w21 We have recently shown in a large series of patients (using ICB waveforms) that the AP pad position is associated with lower transthoracic impedance than the AA configuration.w22 It is likely that this explains the findings of the older monophasic studies, and it is therefore reasonable to recommend the AP electrode positions for monophasic direct current cardioversion.
Several AP pad positions have been described. Some authors feel that a right anterior to left posterior configuration is better where the underlying pathology involves both atria, while a left anterior to posterior configuration is better when the left atrium is primarily affected.w17 The right anterior to left posterior configuration is described,10,12,14 as are the left anterior to right posterior11 and AP midline configurations.13 No data have been published showing superiority of one AP configuration over the other. Finally, our comparison of AP and AA electrode configurations using ICB waveforms demonstrated no difference between these approaches with a modern defibrillator.w22
Internal atrial defibrillation
Internal atrial defibrillation can also be reliably achieved using electrodes placed in the right atrium and distal coronary sinus.w23 This approach has been widely used in patients and subsequently led to the development of a single chamber atrial defibrillator. This device has been shown to be sensitive and specific in the detection of AF and R wave synchronised shocks were delivered appropriately.w24 Follow up of initial implants revealed treatment to be highly efficacious—96% of 227 AF episodes were converted to sinus rhythm.w25 Unfortunately, the frequency of short lived non-treated episodes does not appear to be decreased by this device, and the thromboembolic risk from paroxysmal AF is therefore likely to remain.w26 In addition, a significant number of patients can expect to have the device explanted because of uncontrolled AF.w27 w28
The single chamber atrial defibrillator is now superseded by dual chamber alternatives. Several of these are currently available. They also offer AF preventative pacing, atrial antitachycardia pacing, and atrial defibrillation functions in addition to ventricular defibrillation.w29 However, the efficacy of these devices for the prevention of AF is unproven and long term clinical benefit has not been demonstrated in large numbers of patients. The role of implantable devices for the treatment of AF alone is undefined at present. The place of transvenous atrial defibrillation as a stand alone procedure has not been established in the era of ICB waveforms. However, there may be a use for low energy internal cardioversion for sustained arrhythmia in the electrophysiology laboratory or in selected patient groups (such as the very obese, after cardiac surgery, or in those where external cardioversion fails).
Energy selection for direct current cardioversion
There are two approaches to energy selection for direct current cardioversion. After developing the technique of cardioversion, Lown favoured the escalating energy approach. The rationale for this was to minimise post-shock arrhythmia.w16 This approach also allows cardioversion at the lowest energy for each individual patient and may prevent high cumulative doses in some. Some practitioners prefer to begin the procedure at the highest energy in order to minimise the total number of shocks delivered and the duration of the procedure (including exposure to sedation or anaesthesia) for the majority of patients, while accepting that a proportion will be relatively “overdosed”. The most recent guidelines for AF suggest a starting energy of at least 200 J for attempted cardioversion of AF using monophasic waveforms. This recommendation is made on the basis that energies lower than this are unlikely to be successful.15 However, AF of very short duration (for example, after cardiac surgery) may be amenable to lower energy shocks.14w30
Variable energies have been recommended for defibrillators that deliver impedance compensated biphasic waveforms in the correction of AF. The recommended energy depends on the manufacturer of the device. Published evidence suggests that a shock of 150 J will result in success for ∼80% of patients with these new devices and there does not appear to be a significant additional benefit in the selection of energies above 200 J (table 2). Indeed, this manoeuvre may result in an excess of skin burns.w14 Using an ICB defibrillator, we favour an initial energy of 150 J followed by a 200 J shock.
Table 2.
Success of cumulative shocks for different biphasic devices in cardioversion of AF
| Study | Biphasic device | Success at 70 J (%) | Success at 100 J (%) | Success at 120 J (%) | Success at 150 J (%) | Success at 170 J (%) | Success at 200 J (%) | Success at 360 J (%) |
| Mittal et al10 | Zoll PD-2100 | 68 | – | 85 | 91 | 94 | – | – |
| Page et al11 | HS-XL | – | 60 | – | 77 | – | 90 | – |
| Scholten et alw11 | Zoll M series | – | – | 80 | – | – | 90 | – |
| Walsh et alw22 | HS-XL | 37 | 63 | – | 84 | – | 97 | – |
| Marinsek et alw12 | HS-XL | 28 | 56 | – | 81 | – | 88 | – |
| Neal et alw14 | Lifepak-12 | – | 83 | – | – | – | 96 | 98 |
| Neal et alw14 | Zoll M series | – | 94 | – | – | – | 100 | 100 |
ROLE OF DIRECT CURRENT CARDIOVERSION FOR AF IN THE ERA OF THE RATE CONTROL STRATEGY
The high likelihood of relapse into AF after a successful cardioversion has been recognised for some time. AF has been shown to recur in 88% of patients over 19 months of follow up on “optimal” antiarrhythmic treatment.16 Thus, withdrawal of anticoagulation during periods of sinus rhythm is likely to carry a risk of thromboembolism in a number of patients. Two large clinical studies have recently challenged the rationale of recurrent cardioversion and pharmacological prophylaxis for the maintenance of sinus rhythm in AF patients (table 3).17,18 A total of 4060 patients were enrolled in the AFFIRM study. In the rhythm control group (2033 patients), the antiarrhythmic drugs used were chosen by the treating physician. Attempts to maintain sinus rhythm could include cardioversion as necessary. In the rate control group (2027 patients), drugs that were acceptable in the protocol for this purpose were β blockers, verapamil, diltiazem, digoxin, and combinations of these drugs. The goal for anticoagulation with warfarin was an international normalised ratio (INR) of 2.0–3.0.
Table 3.
Rate control versus rhythm control studies in AF
| Study | Number of patients | Primary end point | Events for rate control group | Events for rhythm control group | p Value |
| AFFIRM17 | 4060 | Overall mortality | 310 | 356 | 0.08 |
| RACE18 | 522 | Composite* | 44 | 60 | 0.11 |
| STAF Pilot19 | 200 | Composite† | 10 | 9 | 0.99 |
*Death from cardiovascular causes, heart failure, thromboembolic complications, bleeding, implantation of a pacemaker, and severe adverse effects of drugs.
†Death, cardiopulmonary resuscitation, cerebrovascular event, and systemic embolism.
AFFIRM, atrial fibrillation follow-up investigation of rhythm management; RACE, rate control versus electrical cardioversion for persistent atrial fibrillation study; STAF, strategies of treatment of atrial fibrillation.
In the rhythm control group, continuous anticoagulation was encouraged but could be stopped at the physician’s discretion if sinus rhythm had apparently been maintained for at least four, and preferably 12, consecutive weeks with antiarrhythmic drug treatment. In the rate control group, continuous anticoagulation was mandated by the protocol. For patients in the rate control group, 35% were in sinus rhythm at five years and over 80% of those in AF had adequate heart rate control. The prevalence of sinus rhythm in the rhythm control group at follow up was 82%, 73%, and 63% at one, three, and five years, respectively. Electrical cardioversion was attempted once during follow up in 368 patients, twice in 214 patients, and three or more times in 187 patients in this group.
There were 356 deaths among the patients assigned to rhythm control treatment and 310 deaths among those assigned to rate control treatment (mortality at five years, 24% and 21%, respectively; hazard ratio 1.15, 95% confidence interval 0.99 to 1.34; p = 0.08). More patients in the rhythm control group than in the rate control group were hospitalised, and there were more adverse drug effects in the rhythm control group. In both groups, the majority of strokes occurred after warfarin had been stopped or when the INR was subtherapeutic. The authors concluded that “management of AF with the rhythm-control strategy offers no survival advantage over the rate-control strategy, and there are potential advantages, such as a lower risk of adverse drug effects, with the rate-control strategy”. Additionally, anticoagulation should be continued in this group of high risk patients.17
The RACE study was smaller, with 522 patients enrolled, but of similar design.18 Patients in the rate control group received oral anticoagulant drugs and rate slowing medication. The rhythm control group had a more rigorous management protocol. These patients initially underwent electrical cardioversion without previous treatment with antiarrhythmic drugs. Thereafter, patients received sotalol. If there was a recurrence within six months, electrical cardioversion was repeated and sotalol was replaced by flecainide or propafenone. If there was a recurrence within six months after the start of this regimen, a loading dose of amiodarone was given, and electrical cardioversion was repeated. The dose of amiodarone was then lowered to 200 mg daily. If sinus rhythm was maintained beyond one month, warfarin could be stopped or substituted by aspirin. A total of 35 thromboembolic complications occurred (14 rate control group and 21 rhythm control group). These occurred more commonly during periods of no or subtherapeutic anticoagulation. The conclusion of the authors was strikingly similar to the AFFIRM study: “rate control is not inferior to rhythm control for the prevention of death and morbidity from cardiovascular causes and may be appropriate therapy in patients with a recurrence of persistent AF after electrical cardioversion”.18 The STAF pilot study provides further support for a rate control strategy.19
Despite these studies, direct current cardioversion is still an important treatment option for patients with symptomatic AF. Many of our patients will complain of lethargy or poor exercise capacity when in AF. It is still reasonable to cardiovert these patients and attempt to maintain sinus rhythm for symptom control. An important lesson from AFFIRM and RACE is that anticoagulation should be continued in those patients who are at risk from thromboembolism. Cardioversion will also be useful where AF is secondary to another condition (for example, postoperative, pneumonia or an exacerbation of thyrotoxicosis, etc), where restoration of sinus rhythm will often be “permanent” after the precipitating event has resolved. Aggressive pursuit of sinus rhythm can no longer be justified in asymptomatic AF. Where the arrhythmia is symptomatic but refractory, alternative strategies such as atrioventricular nodal ablation and pacing should be considered.
AF and direct current conversion: key points.
Atrial fibrillation (AF) is mainly re-entry associated with multiple propagating wavelets in the atria probably initiated by ectopic foci originating from the pulmonary veins
Direct current cardioversion is an important treatment for patients with symptomatic AF
Impedance compensated biphasic defibrillators are replacing monophasic defibrillators since successful cardioversion is achieved with lower energies and fewer shocks
The anteroposterior electrode configuration appears optimal for monophasic direct current cardioversion (lower transthoracic impedance). There is no difference between the anteroposterior and antero-apical electrode configurations when impedance compensated biphasic waveforms are used
Where monophasic waveforms are used, the first shock should be 200 J except for AF of very short duration
Where biphasic waveforms are used, the first shock should be 150 J
A rate control strategy (pharmacological including anticoagulant treatment) is satisfactory in those where AF has recurred or in those with chronic AF
FUTURE DEVELOPMENTS IN DIRECT CURRENT CARDIOVERSION
Biphasic defibrillators are replacing their monophasic counterparts for this procedure (monophasic devices are no longer available from most manufacturers). In the next few years warfarin is likely to be superseded by alternative anticoagulants. New antiarrhythmic agents may revitalise the rhythm control strategy in the future if these drugs have comparable efficacy to amiodarone with safer side effect profiles. The role of implantable devices is still under investigation. Hybrid devices that maintain sinus rhythm by multisite atrial pacing and facilitate recurrent internal direct current cardioversion by the delivery of radiofrequency energy20 have potential for some patients with AF.
Supplementary Material
REFERENCES
- 1.Jalife J. Ventricular fibrillation: mechanisms of initiation and maintenance. Annu Rev Physiol 2000;62:25–50. [DOI] [PubMed] [Google Scholar]
- 2.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 2002;54:204–216. ▸ Review of the mechanisms of atrial fibrillation. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 4.Gray RA, Ayers GM, Jalife J. Video imaging of atrial defibrillation in the sheep heart. Circulation 1997;95:1038–47. ▸ Examination of the effects of atrial defibrillation using potentiometric dyes. [DOI] [PubMed] [Google Scholar]
- 5.Ideker RE, Wolf PD, Tang ASL. Mechanisms of defibrillation. In: Tacker WA, ed. Defibrillation of the heart. St Louis, Missouri: Mosby-Year Book, Inc, 1994:15–45.
- 6.Zipes DP, Fischer J, King RM, et al. Termination of ventricular fibrillation in dogs by depolarizing a critical amount of myocardium. Am J Cardiol 1975;36:37–44. ▸ Original demonstration of the critical mass theory in ventricular defibrillation. [DOI] [PubMed] [Google Scholar]
- 7.Ideker RE, Chattipakorn N, Gray RA. Defibrillation mechanisms: the parable of the blind men and the elephant. J Cardiovasc Electrophysiol 2000;11:1008–13. ▸ Authoritative review of mechanisms of defibrillation. [DOI] [PubMed] [Google Scholar]
- 8.Kwaku KF, Dillon SM. Shock-induced depolarization of refractory myocardium prevents wave-front propagation in defibrillation. Circ Res 1996;79:957–73. [DOI] [PubMed] [Google Scholar]
- 9.Sims JJ, Miller AW, Ujhelyi MR. Disparate effects of biphasic and monophasic shocks on postshock refractory period dispersion. Am J Physiol 1998;274:H1943–9. ▸ Demonstration of how biphasic shocks have different effects on refractory period dispersion compared to monophasic shocks. [DOI] [PubMed] [Google Scholar]
- 10.Mittal S, Ayati S, Stein KM, et al. Transthoracic cardioversion of atrial fibrillation. Comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation 2000;101:1282–7. ▸ Study demonstrating the superiority of rectilinear biphasic shocks over their monophasic counterparts for direct current cardioversion of AF. [DOI] [PubMed] [Google Scholar]
- 11.Page RL, Kerber RE, Russell JK, et al. for the BiCard Investigators. Biphasic versus monophasic shock waveform for conversion of atrial fibrillation. J Am Coll Cardiol 2002;39:1956–63. ▸ Demonstrated that direct current cardioversion requires fewer shocks with biphasic waveforms and also showed that skin burns occur less commonly with biphasic waveforms. [DOI] [PubMed] [Google Scholar]
- 12.Botto GL, Politi A, Bonini W, et al. External cardioversion of atrial fibrillation: role of paddle position on technical efficacy and energy requirements. Heart 1999;82:726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirchhof P, Eckardt L, Loh P, et al. Anterior-posterior versus anterior-lateral electrode positions for external cardioversion of atrial fibrillation: a randomized controlled trial. Lancet 2002;360:1275–9. [DOI] [PubMed] [Google Scholar]
- 14.Mathew TP, Moore A, McIntyre M, et al. Randomised comparison of electrode positions for cardioversion of atrial fibrillation. Heart 1999;81:576–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC Guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the European Society of Cardiology committee for practice guidelines and policy conferences (committee to develop guidelines for the management of patients with atrial fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. Eur Heart J 2001;22:1852–923. ▸ Excellent review of all aspects of AF, with current recommendations for management. [DOI] [PubMed] [Google Scholar]
- 16.Israel CW, Gronefeld G, Ehrlich JR, et al. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol 2004;43:47–52. [DOI] [PubMed] [Google Scholar]
- 17.The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33. ▸ Large clinical trial showing that a rate control strategy is not inferior to a rhythm control strategy. [DOI] [PubMed] [Google Scholar]
- 18.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;377:1834–40. ▸ Further data showing that a rate control strategy is not inferior to a rhythm control strategy. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson J, Miketic S, Windeler J, et al. for the STAF Investigators. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation. The strategies of treatment of atrial fibrillation (STAF) study. J Am Coll Cardiol 2003;41:1690–6. [DOI] [PubMed] [Google Scholar]
- 20.Manoharan G, Evans N, Kidwai B, et al. Novel passive implantable atrial defibrillator using transcutaneous radiofrequency energy transmission successfully cardioverts atrial fibrillation. Circulation 2003;108:1382–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.