Abstract
Underlying mechanisms in the development of atrial flutter or intra-atrial re-entry tachycardia in patients with structural cardiac abnormalities remain poorly defined. The right atrial myocardium from two patients with congenital heart disease was evaluated, of whom one presented with severe right atrial dilation and arrhythmia and the other with a normal right atrium, to assess whether increased right atrial pressure and volume overload give rise to sarcolemmal alteration. N-terminus dystrophin staining in the atrium from the patient who had undergone a Fontan procedure showed a normally distributed but significantly reduced staining signal compared with the second patient. This is the first report that patients with severe right atrial dilation and atrial flutter have marked reduction in atrial dystrophin expression.
Keywords: atrial flutter, dystrophin, Fontan circuit
Underlying mechanisms in the development of atrial flutter or intra-atrial re-entry tachycardia in patients with structural cardiac abnormalities remain poorly defined.
It has been shown that in cardiomyocytes from mice lacking dystrophin (mdx), inactivation of the L-type Ca2+ channels is reduced, the voltage dependence of activation is shifted towards a more positive potential, and β adrenergic stimulation is increased leading to a prolonged QT interval in mdx mice.1 These results suggest a role for dystrophin in regulating the activity of the cardiac L-type Ca2+ channel and a potential mechanism for cardiac arrhythmias in a structurally altered myocardium. Therefore, we evaluated the right atrial myocardium from two patients with congenital heart disease, one of whom presented with severe right atrial dilatation and arrhythmia and the other with a normal right atrium, to assess whether increased right atrial pressure and volume overload give rise to sarcolemmal alteration.
CASE REPORT
The first patient was an 11 year old boy who had undergone a total cavopulmonary communication for tricuspid atresia, after which he developed severe right atrial dilation and atrial flutter and subsequently required revision of his Fontan circuit and a Maze procedure. The second patient (control atrium) with no history of right atrial disease or arrhythmias underwent repair of an aortopulmonary window. Informed consent was obtained from the children’s families, and the study was approved by the internal review board of Baylor College of Medicine. At the time of surgery a 3–5 mm specimen of right atrial tissue from the lateral wall was resected in each patient and immediately snap frozen in liquid nitrogen. The samples were stained with the DYS3 antibody to the N-terminus of dystrophin (Novocastra, Newcastle, UK). The primary antibody was diluted 1:20 in 1 × phosphate buffered saline and applied to each section for one hour at room temperature. After three quick washings in 1 × phosphate buffered saline, the slides were incubated for 30 minutes at room temperature with the secondary antibody, a mouse IgG fluorescein-isothiocyanate conjugated antibody (Novocastra), diluted 1:400, then mounted, as previously described.2
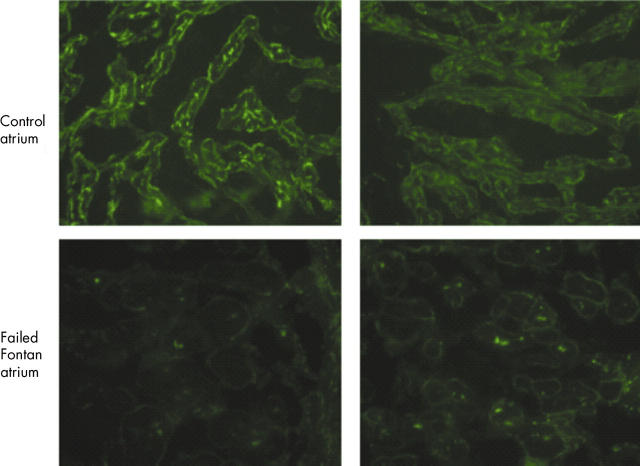
The immunohistochemical analysis showed differential dystrophin expression between the normal and dilated right atrium. N-terminus dystrophin staining in the atrium from the patient with a dilated atrium showed a normally distributed but significantly reduced staining signal compared with the control atrium (fig 1). Although myocardial dystrophin has previously been reported to undergo remodelling in patients with end stage cardiomyopathy in both right and left ventricles,2,3 this is the first report that patients with severe right atrial dilation and atrial flutter have greatly reduced dystrophin expression within their atria.3,4
Figure 1.
Dystrophin expression in a patient with severe right atrial disease and arrhythmia (Failed Fontan atrium) compared with a patient with a normal right atrial structure and function (Control atrium). There is a significant reduction in staining in the N-terminus portion of dystrophin in the diseased atrium from the patient with intra-atrial re-entry tachycardia compared with the control patient.
DISCUSSION
The dystrophin abnormality in the right atrium may be induced by right atrial mechanical overload and wall tension, which can lead to altered L-type Ca2+ channels with subsequent risk of atrial flutter, since the cardiac voltage gated L-type Ca2+ channel (CaV1.2) mediates the Ca2+ current that is responsible for the plateau phase of the cardiac action potential and for initiating contraction. In addition, decreasing wall tension within the atrium through Fontan revision may not only induce reverse remodelling of the myocardium, as it does after treatment with a left ventricular assist device,2 but also possibly reduce the recurrence of atrial flutter by restoring the correct association between dystrophin and CaV1.2.
REFERENCES
- 1.Sadeghi A, Doyle AD, Johnson BD. Regulation of the cardiac L-type Ca2+ channel by the actin-binding proteins alpha-actinin and dystrophin. Am J Physiol Cell Physiol 2002;282:C1502–11. [DOI] [PubMed] [Google Scholar]
- 2.Vatta M, Stetson SJ, Perez-Verdia A, et al. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet 2002;359:936–41. [DOI] [PubMed] [Google Scholar]
- 3.Vatta M, Stetson SJ, Jimenez S, et al. Molecular normalization of dystrophin in the failing left and right ventricle of patients treated with either pulsatile or continuous flow-type ventricular assist devices. J Am Coll Cardiol 2004;43:811–7. [DOI] [PubMed] [Google Scholar]
- 4.Anastasi G, Cutroneo G, Trimarchi F, et al. Sarcoglycans in human skeletal muscle and human cardiac muscle: a confocal laser scanning microscope study. Cells Tissues Organs 2003;173:54–63. [DOI] [PubMed] [Google Scholar]