Abstract
Objective: To test the hypothesis that atrial fibrillation (AF) is associated with changes in the expression of connexins 40 and 43 in the left atrium with more pronounced changes in mitral valve disease than in lone AF.
Methods: Protein concentrations of connexin 40 and connexin 43 were analysed in left atrial tissue of patients undergoing cardiac surgery. One group of patients had lone AF (n = 41), one group had AF and mitral valve repair (n = 36), and one group in sinus rhythm served as controls (n = 15).
Results: Western blot analysis of connexin 40 and connexin 43 expression showed an increase of both gap junctional proteins (connexin 43 > connexin 40) in patients with AF of all forms compared with patients in sinus rhythm (p = 0.01 and p = 0.011, respectively). Subgroup analysis showed increased concentrations of connexin 40 in lone AF and AF with mitral valve disease compared with sinus rhythm (p = 0.06 and p = 0.029, respectively), whereas the same analysis for connexin 43 reached significance only in the mitral valve disease group (p = 0.031). No differences in connexin 40 and connexin 43 expression were detectable between lone AF and AF with mitral valve disease. Within the groups connexin 40 and connexin 43 expression did not differ between patients with paroxysmal AF and patients with chronic AF.
Conclusion: The present study shows for the first time that AF can induce changes in the left atrium with increased connexin expression. Furthermore, no systematic differences between patients with paroxysmal and chronic AF were detected.
Keywords: atrial fibrillation, connexin, gap junctions, human atrium
Atrial fibrillation (AF) is very common in patients with mitral valve disease (MVD), although in many cases no specific cause of AF can be found—so called lone AF. AF of whatever cause leads to alterations in the electrophysiological properties of the atrial tissue, referred to as electrical remodelling, with a reduced effective refractory period, action potential duration, and conduction velocity, thereby increasing the stability of re-entrant circuits and promoting its own perpetuation.1–4 Conduction depends on cell to cell communication, which is determined by gap junction channels. In cardiac tissue connexins 40, 43, and 45 have been detected, with connexins 40 and 43 being the main components of the atrial gap junctions.4–8 Given the major role of these connexins in electrical conduction, and taking into consideration the observed pronounced electrical remodelling in AF, we proposed to find differences in connexin 40 and 43 expression between patients with AF an patients in sinus rhythm (SR). So far there are only limited and contradictory data on the role of connexins in electrical remodelling, especially in AF and mostly in the right atrial tissue.9–21 To test the hypothesis that AF is associated with changes in the expression of connexins 40 and 43 in the left atrium (as the typical origin of AF) with more pronounced changes in MVD than in lone AF, we analysed connexin 40 and connexin 43 protein expression in atrial tissue of patients with SR, lone AF, and AF in MVD. We also compared paroxysmal (PAF) with chronic AF (CAF).
METHODS
Patients
The study group comprised patients undergoing cardiac surgery for lone AF (lone AF group, n = 41: 21 with PAF and 20 with CAF), for mitral valve repair or replacement in conjunction with intraoperative radiofrequency ablation of AF (MVD with AF group, n = 36: seven with PAF, 29 with CAF), and patients in SR undergoing cardiac surgery for coronary artery bypass grafting (CABG), valve replacement, or valve repair (detailed in table 1) (SR group, n = 15: eight with CABG or aortic valve replacement as the control group for the lone AF group, seven with SR and additional MVD). The majority of operations were minimally invasive. All tissue samples were obtained at the same stage of the surgical procedure during extracorporeal circulation.
Table 1.
Summary of patient data
| Lone AF group (n = 41) | MVD group (n = 36) | Control group (n = 15) | p Value | |
| PAF | 21 | 7 | 0 | |
| CAF | 20 | 29 | 0 | |
| Cardiac surgery | IRAAF | MVR+IRAAF | MVR 6, MVR+CABG 1, CABG 6, AVR+CABG 2 | |
| Age (years) | 50 (12) | 63.5 (10) | 61 (8) | <0.05*, NS† |
| LVEF (%) | 60 (7) | 56 (15) | 55 (15) | NS |
| Left atrium (mm) | 43 (6) | 55 (11) | 44 (7) | <0.05*, NS† |
Data are mean (SEM) or number.
AF, atrial fibrillation; AVR, aortic valve repair or replacement; CABG, coronary artery bypass grafting; CAF, chronic atrial fibrillation; IRAAF, intraoperative radiofrequency ablation of atrial fibrillation (placement of linear lesions within the left atrium22); LVEF, left ventricular ejection fraction; MVD, mitral valve disease; MVR, mitral valve repair or replacement; NS, not significant; PAF, paroxysmal atrial fibrillation.
*Lone AF versus MVD; †versus control.
Patients of the control group were matched to the AF groups according to age, left atrial size, and left ventricular function. Patients were enrolled in the study only if they had preserved left ventricular function and, in the lone AF group, if they had a left atrial size of ⩽ 45 mm as assessed by echocardiography. Patients with impaired left ventricular function or very enlarged left atria (> 55 mm) and additional cardiac diseases such as coronary artery disease or aortic valve disease were not included in the study group. Left ventricular function had to be normal in the control group. The surgical procedure and the concept of intraoperative ablation of AF have been described in detail previously.22 All patients gave informed consent. The institutional ethics committee approved the study. The investigation conforms with the principles outlined in the Declaration of Helsinki. Table 1 summarises clinical data of the patients.
Tissue preparation
During cardiac surgery for intraoperative ablation or mitral valve repair, left atrial tissue samples were obtained from the left sided atriotomy. In the control group only in patients undergoing CABG (n = 8), samples were taken from the right atrial appendage. The material was immediately frozen in liquid nitrogen and stored at −80°C until further investigation.
Western blot analysis
For protein extraction frozen atrial tissue samples were homogenised in hydroxyethylpiperazine-ethanesulfonic acid (HEPES) buffer with the addition of protease inhibitors (0.5 mg/ml leupeptin, 10 µg/ml aprotinin) and 1 mM phenylmethylsulfonyl fluoride (Boehringer, Mannheim, Germany). A total of 50 µg protein was separated on a 12% sodium dodecyl sulfate polyacrylamide gel and blotted on to nitrocellulose membranes (Roth, Karlsruhe, Germany) with a tank blotting system (BioRad, Munich, Germany). Membranes were blocked with 5% milk powder (Roth) in tris buffered saline with 0.5% Tween 20 for one hour. After washing (three times for five minutes in Tween 20 and tris buffered saline) membranes were incubated with the primary antibodies rabbit anti-human connexin 40 and rabbit anti human connexin 43 (Alpha Diagnostics, San Antonio, Texas, USA) and mouse anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hytest, Turku, Finland) for two hours. After a second washing step, membranes were incubated with secondary antibodies conjugated with horseradish peroxidase for one hour, goat anti-rabbit IgG (Dianova, Hamburg, Germany), or rabbit anti-mouse IgG (Sigma, Deisenhofen, Germany). Membranes were washed three times in Tween 20 and tris buffered saline and subsequently developed with Super Signal Reagent (Pierce, Rockford, Illinois, USA). The specificity of the antibodies has been determined previously.21
Densitometric analysis
Immunoblots were exposed to x ray film (Eastman Kodak Co), developed, and analysed by ONE-Dscan 1.0 Software (Scanalytics, Los Angeles, California, USA). The relative amount of connexin in each sample was determined by comparing the grey scale values of connexin with GAPDH. The housekeeping protein GAPDH was used to assure that the same amounts of cellular protein were assessed in each sample. To avoid measurement mistakes by different x ray exposure all values were normalised to the grey scale value of an internal standard.
Statistical analysis
All data are represented as mean (SEM). Statistical evaluation was done by two way multivariate analysis of variance. Values of p < 0.05 were considered significant. All measurements were done in triple and mean values were used for further analysis.
RESULTS
Patients
Table 1 presents clinical data of the patients. Patients in the lone AF group were younger and had smaller left atrial sizes than patients in the MVD group (p < 0.05). Left ventricular function was similar in both groups. Control patients were matched according to age and left atrial size. There were no significant differences in clinical data between the control group and the study groups.
Influence of AF on connexin 40 and connexin 43 expression
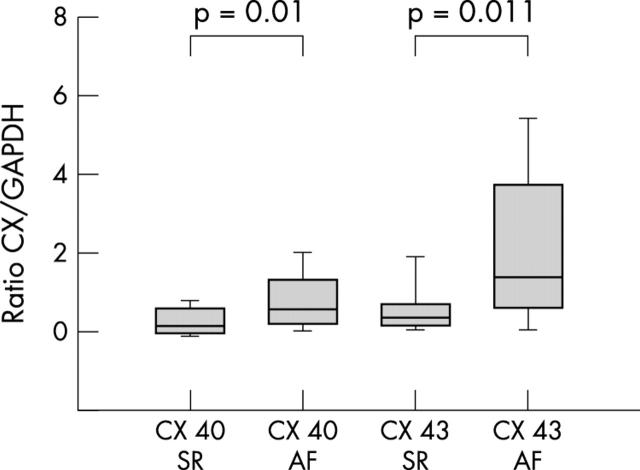
Western blot analysis of connexin 40 and connexin 43 expression presented a clear increase of both gap junctional proteins in patients with AF of either cause (lone AF and MVD, n = 77) compared with patients in SR with and without MVD (n = 15). The protein concentration of connexin 40 in left atrial tissue was slightly but significantly increased (p = 0.01) in patients with AF. More clearly, connexin 43 protein was significantly enhanced (p = 0.011) in patients with AF compared with all patients in SR (fig 1).
Figure 1.
Connexin 40 (Cx 40) and connexin 43 (Cx 43) protein concentrations in patients with atrial fibrillation (AF) compared with sinus rhythm (SR). Concentrations are expressed as the ratio of Cx to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping protein. All data are mean (SEM).
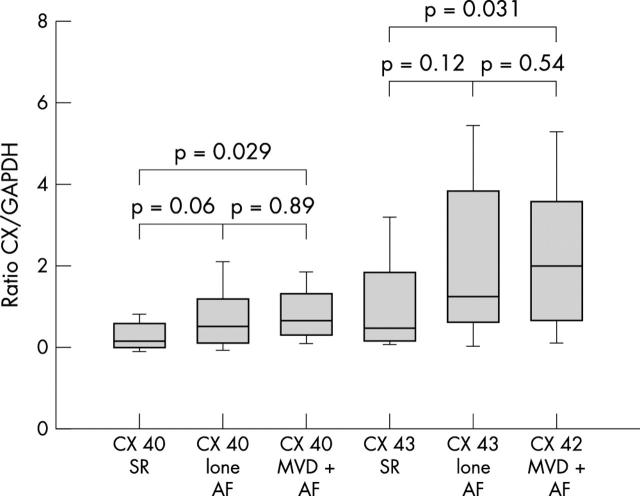
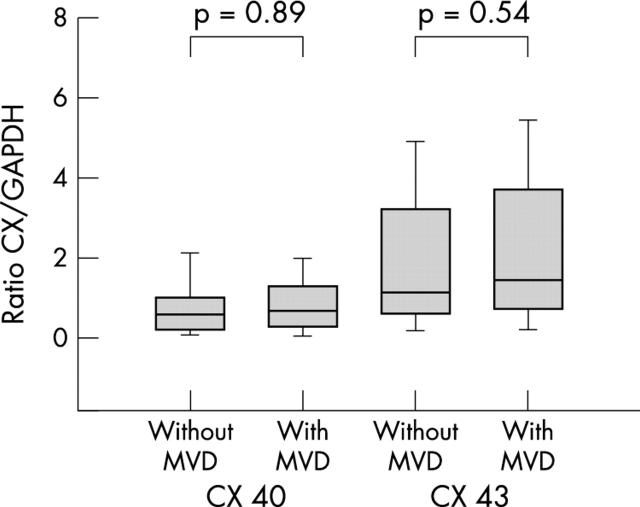
Next we analysed patient subgroups. Comparing connexin 40 expression between SR control patients with lone AF, we found an almost significant increase in lone AF (p = 0.06). In the MVD and AF group the increase in connexin 40 compared with the SR group was significant (p = 0.029). There were no differences in connexin 40 concentration in left atrial tissue between patients with lone AF and patients with MVD and AF (p = 0.89) (fig 2).
Figure 2.
Cx 40 and Cx 43 protein concentrations in patients with lone AF and AF with mitral valve disease (MVD+AF) compared with SR. Concentrations are expressed as the ratio of Cx to GAPDH and are given as mean (SEM).
The same comparisons for connexin 43 expression showed an increase (though not significant) in the lone AF group and a significant increase in the MVD with AF group compared with the SR group (p = 0.12 and p = 0.031, respectively) (fig 2). There were also no significant differences in connexin 43 expression between the lone AF and the MVD with AF group (p = 0.54).
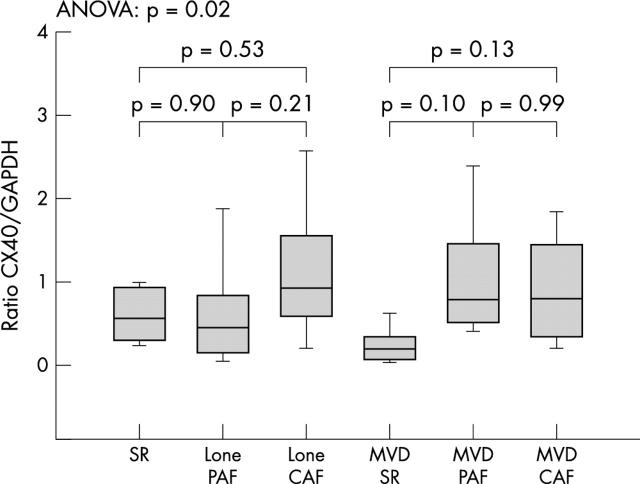
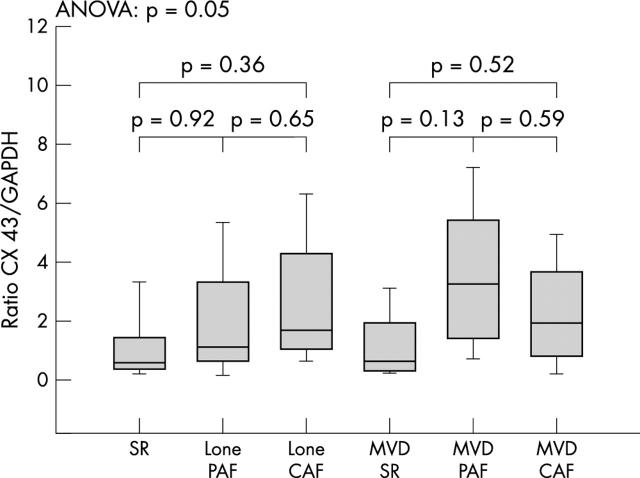
To investigate the influence of the PAF and CAF forms of AF on the protein expression of connexin 40 and connexin 43 we compared patients with PAF and CAF within the groups. No significant differences in either connexin 40 or connexin 43 expression were found when comparing patients with PAF versus CAF within the lone AF and the MVD group (figs 3 and 4).
Figure 3.
Cx 40 expression in paroxysmal (PAF) and chronic atrial fibrillation (CAF) in the lone AF and the MVD+AF groups. Concentrations are expressed as the ratio of Cx to GAPDH and given as mean (SEM). ANOVA, analysis of variance.
Figure 4.
Cx 43 expression in PAF and CAF in the lone AF and the MVD+AF groups compared with SR control patients. Concentrations are expressed as the ratio of Cx to GAPDH and given as mean (SEM).
Influence of MVD on connexin 40 and connexin 43 expression
Lastly, to determine the influence of MVD on connexin expression in general, we compared all patients without MVD (SR and lone AF, n = 49) with all patients with MVD (MVD and SR, and MVD and AF, n = 43) and did not detect any changes in either connexin 40 or connexin 43 protein expression (fig 5).
Figure 5.
Cx 40 and Cx 43 protein concentrations in patients without MVD (including all patients with lone AF and SR controls without MVD) compared with patients with MVD (AF and SR group).
DISCUSSION
The present study shows that AF is accompanied by significant changes in the protein expression of connexins 40 and 43 in the left atrium (connexin 43 > connexin 40). Subgroup analysis, however, showed a trend towards increased concentrations of connexin 40 in lone AF and significantly increased concentrations of connexin 40 protein in MVD and AF. Differences in connexin 43 expression were significant only in the MVD and AF subgroup. Interestingly, connexins 40 and 43 changed to a similar extent in patients with lone AF and in patients with AF with MVD, suggesting that AF alone may be enough to lead to changes of the atrium with increased expression of gap junctional proteins and that MVD does not induce further changes. Furthermore, no systematic differences between patients with PAF and patients with CAF were detected.
To our knowledge this is the first study of changes in connexin 40 and connexin 43 expression in human lone AF in the left atrium. So far only a limited number of studies on connexin expression in human AF are available. All of them investigated only patients with advanced structural heart disease with an indication for cardiac surgery.14,15,17,20,23
Regarding connexin 40 our data are in close accordance with data presented by Polontchouk et al,14 who observed a similar increase in connexin 40 in the right atrium of patients with CAF. Dupont et al15 also found significantly higher connexin 40 concentration in patients susceptible to postoperative AF, although this arrhythmia and “naturally occurring” AF are probably caused by different pathophysiological mechanisms. On the other hand, Kostin et al17 described a significant decrease of connexin 40 in the right atrial appendages of patients with AF and a slight but non-significant increase in the right atrial free wall of the same patients. Recently Nao et al19 showed a significant reduction of connexin 40 protein and mRNA concentrations in the right atrial tissue of patients with MVD and AF compared with normal SR and MVD and normal controls (patients undergoing CABG), but they described a significant increase in serine phosphorylated connexin 40 in MVD and AF. In the goat model of pacing induced AF no quantitative changes in connexin 40 were found but there was a pronounced change in the distribution of connexin 40 from end to end junctions to the lateral wall.13,20
The available data on connexin 43 are also inconclusive, since Kostin et al17 described significant decreases in connexin 43 in patients with AF, whereas Polontchouk et al,14 Dupont et al,15 and Nao et al19 in human material and van der Velden et al18,21 in goats did not find any quantitative change in connexin 43 with AF. However, the source of the tissue (right or left atrium) should be taken into account. Only Elvan et al12 observed an increase in connexin 43 in pacing induced AF, which returned to normal after linear radiofrequency ablation within the right atrium. Several attempts have been undertaken either surgically or by catheter techniques to cure AF by linear ablation especially within the left atrium.22,24
The functional consequence of our findings is not clear at the moment, since we do not know whether the expressed connexin 40 and connexin 43 proteins form functional gap junction channels and, if so, what kind of electrical alterations are caused by a higher number of gap junction channels. It is known from animal studies with knock out models that changes in connexin 40 are accompanied by changes in atrial electrical coupling and susceptibility to AF,25–28 whereas changes in connexin 43 lead to disturbances of ventricular electrical coupling.28,29 In cardiac diseases altering ventricular function, changes in connexin 43 have been observed. A common feature in ventricular function disturbance is a reduction in connexin 43 (the main ventricular connexin) and a change in gap junction distribution and size with an increase in side to side coupling and a decrease in end to end connections. Changes in gap junctional organisation or in the amount of connexin 43 are associated with an increased risk of arrhythmias in the diseased ventricle. In the atrium higher connexin 40 concentrations are associated with a higher risk of developing AF after cardiac surgery.11–13,30–37 According to these data one would expect to find only changes in connexin 40, the main atrial connexion, and not necessarily changes in connexin 43. However, in our study we clearly detected differences in connexin 40 and connexin 43 protein expression in patients with AF. If the increased expression of connexin 40 and connexin 43 protein leads to functioning gap junction channels both longitudinally and laterally at the cell wall, one would expect a change in the biophysical properties of the cells as shown by Polontchouk et al.38 Further studies to examine the connexin distribution and function in the atria, and cell culture and animal studies analysing the electrophysiological properties of the changed gap junction channels, are needed.
Regulation of gap junctional expression, distribution, and function is still under investigation. A lot of factors such as phosphorylation and dephosphorylation, voltage gradients, and many signalling pathways seem to be involved.5–7,38 Gap junction channels have a very short half life and are therefore prone to regulatory mechanisms.6,7,33 Recently it was shown that the renin–angiotensin system is involved in the regulation of connexin expression through the AT1 receptor, hinting that altered connexin expression and arrhythmogenesis are linked to various cardiac diseases accompanied by changes in the renin–angiotensin system.6,33,34,38–40 Involvement of the renin–angiotensin system may allow new treatment options for the many patients with AF. The first clinical observations have already been made of reduced AF recurrences in patients treated with angiotensin converting enzyme inhibitors.
Study limitations
Our study had some limitations mainly caused by tissue sample availability. Because of ethical and surgical concerns it was not possible to analyse left atrial tissue from healthy patients in SR without MVD. So far there are no conclusive data on differential connexin expression in the left and right atrium. Therefore, we hypothesised that the distribution of connexins 40 and 43 and their changes in pathological processes are similar in left and right atrial tissue. However, according to the data of this study connexin 40 and connexin 43 protein expression needs to be compared in the left and right atrium; we are investigating this comparison in our laboratory.
Conclusion
Our study showed a significant up regulation of connexin 40 and even more of connexin 43 protein expression in the left atrial tissue of patients with AF without and with MVD. Changes were of about the same magnitude in lone AF and in AF with MVD with no significant differences between PAF and CAF. Changes in gap junctional protein expression may be important in the electrical and morphological remodelling processes in AF. A better understanding of the pathophysiological mechanisms in the development and perpetuation of AF will improve the management of arrhythmia.
Abbreviations
AF, atrial fibrillation
CABG, coronary artery bypass grafting
CAF, chronic atrial fibrillation
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
HEPES, hydroxyethylpiperazine-ethanesulfonic acid
MVD, mitral valve disease
PAF, paroxysmal atrial fibrillation
SR, sinus rhythm
REFERENCES
- 1.Wijffels MCEF, Kirchhof CJHJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation 1995;92:1954–68. [DOI] [PubMed] [Google Scholar]
- 2.Wijffels MCEF, Kirchhof CJHJ, Dorland R, et al. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats:roles of neurohumoral changes, ischemia, atrial stretch, and high rate of atrial activation. Circulation 1997;96:3710–20. [DOI] [PubMed] [Google Scholar]
- 3.Goette A, Honeycutt C, Landberg JJ. Electrical remodeling in atrial fibrillation: time course and mechanisms. Circulation 1996;94:2968–74. [DOI] [PubMed] [Google Scholar]
- 4.Jalife J, Morley GE, Vaida D. Connexins and impulse propagation in the mouse heart. J Cardiovasc Electrophysiol 1999;10:1649–63. [DOI] [PubMed] [Google Scholar]
- 5.Berul CI. A gap in understanding the connection between connexins and cardiac conduction. J Cardiovasc Electrophysiol 1999;10:1376–9. [DOI] [PubMed] [Google Scholar]
- 6.Van Veen TAB, van Rijen HVM, Opthof T. Cardiac gap junction channels: modulation of expression and channel properties. Cardiovasc Res 2001;51:217–9. [DOI] [PubMed] [Google Scholar]
- 7.Saffitz JE, Laing JG, Yamada KA. Connexin expression and turnover: implications for cardiac excitability. Circ Res 2000;86:723–8. [DOI] [PubMed] [Google Scholar]
- 8.Kanagaratnam P, Rothery S, Patel P, et al. Relative expression of immunolocalized connexin 40 and 43 correlates with human atrial conduction properties. J Am Coll Cardiol 2002;39:116–23. [DOI] [PubMed] [Google Scholar]
- 9.Kanagaratnam P, Severs NJ, Peters NS. The relationship between conduction, activation pattern and quantity of immunoreactive connexin in chronic human atrial fibrillation (abstract). Eur Heart J 2000;21:240. [Google Scholar]
- 10.Takeuchi S, Akita T, Takagishi Y, et al. Disorganization of gap junction coupling in human chronic atrial fibrillation (abstract). Eur Heart J 2000;21:240. [Google Scholar]
- 11.Spach MS, Starmer CF. Altering the topology of gap junctions a major therapeutic target for atrial fibrillation. Cardiovasc Res 1995;30:336–44. [DOI] [PubMed] [Google Scholar]
- 12.Elvan A, Huang XD, Pressler ML, et al. Radiofrequency catheter ablation of the atria eliminates pacing-induced sustained atrial fibrillation and reduces connexin 43 in dogs. Circulation 1997;96:1675–85. [DOI] [PubMed] [Google Scholar]
- 13.Van der Velden HMW, Van Kempen MJA, Wijffels MCEF, et al. Altered pattern of connexin 40 distribution in persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol 1998;9:596–607. [DOI] [PubMed] [Google Scholar]
- 14.Polontchouk L, Haeflinger JA, Ebelt B, et al. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol 2001;38:883–91. [DOI] [PubMed] [Google Scholar]
- 15.Dupont E, Ko YS, Rothery S, et al. The gap-junctional protein connexin 40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation 2001;103:842–9. [DOI] [PubMed] [Google Scholar]
- 16.Van der Velden, Wilders R, Jongsma HJ. Atrial fibrillation-induced gap junctional remodeling. J Am Coll Cardiol 2002;39:1709–10. [DOI] [PubMed] [Google Scholar]
- 17.Kostin S, Klein G, Szalahy Z, et al. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res 2002;54:361–79. [DOI] [PubMed] [Google Scholar]
- 18.Van der Velden HMW, Jongsma HJ. Cardiac gap junctions and connexins: their role in atrial fibrillation and potential as therapeutic targets. Cardiovasc Res 2002;54:270–9. [DOI] [PubMed] [Google Scholar]
- 19.Nao T, Ohkusa T, Hisamatsu Y, et al. Comparison of expression of connexin in right atrial myocardium in patients with chronic atrial fibrillation versus those in sinus rhythm. Am J Cardiol 2003;91:678–83. [DOI] [PubMed] [Google Scholar]
- 20.Ausma J, van der Velden HM, Lenders MH, et al. Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation 2003;107:2051–8. [DOI] [PubMed] [Google Scholar]
- 21.Van der Velden HMW, Ausma J, Rook MB, et al. Gap junctional remodeling in relation to stabilization of atrial fibrillation. Cardiovasc Res 2000;46:476–86. [DOI] [PubMed] [Google Scholar]
- 22.Kottkamp H, Hindricks G, Autschbach R, et al. Specific linear left atrial lesions in atrial fibrillation: intraoperative radiofrequency ablation using minimally invasive surgical techniques. J Am Coll Cardiol 2002;40:481–3. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi S, Akita T, Takagishi Y, et al. Disorganization of gap junction coupling in human chronic atrial fibrillation [abstract]. Eur Heart J 2000;21:240. [Google Scholar]
- 24.Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104:2539–44. [DOI] [PubMed] [Google Scholar]
- 25.Verheule S, van Batenburg CAJAC, Coenjaerts FEJ, et al. Cardiac conduction abnormalities in mice lacking the gap junction protein connexin 40. J Cardiovasc Electrophysiol 1999;10:1380–9. [DOI] [PubMed] [Google Scholar]
- 26.Hagendorff A, Schumacher B, Kirchhoff S, et al. Conduction disturbances and increased atrial vulnerability in connexin40-deficient mice analysed by transesophageal stimulation. Circulation 1999;99:1508–15. [DOI] [PubMed] [Google Scholar]
- 27.Morley GE, Vaidya D, Samie FH, et al. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol 1999;10:1361–75. [DOI] [PubMed] [Google Scholar]
- 28.Olgin JE, Verheule S. Transgenic and knockout mouse models of atrial arrhythmias. Cardiovasc Res 2002;54:280–6. [DOI] [PubMed] [Google Scholar]
- 29.Peters NS, Coromilas J, Severs NJ, et al. Disturbed connexin 43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation 1997;95:988–96. [DOI] [PubMed] [Google Scholar]
- 30.Kaprielian RR, Gunning M, Dupont E, et al. Downregulation of immunodetectable connexin 43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation 1998;97:651–60. [DOI] [PubMed] [Google Scholar]
- 31.Severs NJ, Rothery S, Dupont E, et al. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech 2001;52:301–22. [DOI] [PubMed] [Google Scholar]
- 32.Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res 2000;86:1193–7. [DOI] [PubMed] [Google Scholar]
- 33.Emdad L, Uzzaman M, Takagishi Y, et al. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: prevention by angiotensin II type 1 receptor blockade. J Mol Cell Cardiol 2001;33:219–31. [DOI] [PubMed] [Google Scholar]
- 34.Dupont E, Matsushita T, Kaba RA, et al. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol 2001;33:359–71. [DOI] [PubMed] [Google Scholar]
- 35.Huang XD, Sandusky GE, Zipes DP. Heterogenous loss of connexin 43 in ischemic dog hearts. J Cardiovasc Electrophysiol 1999;10:79–91. [DOI] [PubMed] [Google Scholar]
- 36.Sepp R, Severs NJ, Gourdie RG. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart 1996;76:412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pimentel RC, Yamada KA, Kleber AG, et al. Autocrine regulation of myocyte Cx 43 expression by VEGF. Circ Res 2002;90:671–7. [DOI] [PubMed] [Google Scholar]
- 38.Polontchouk L, Ebelt B, Jackels M, et al. Chronic effect of endothelin 1 and angiotensin II on gap junctions and intercellular communication in cardiac cells. FASEB Lett 2002;16:87–9. [DOI] [PubMed] [Google Scholar]
- 39.Van der Velden HMW, Ausma J, Rook MB, et al. Gap junction remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc Res 2000;46:476–86. [DOI] [PubMed] [Google Scholar]
- 40.Boldt A, Wetzel U, Garbade J, et al. Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. J Am Coll Cardiol 2003;42:1785–92. [DOI] [PubMed] [Google Scholar]