Weight loss greater than 6% independently correlates with reduced survival in patients with congestive heart failure (CHF).1 Cardiac cachexia is a gradual and graded process, with wasting affecting muscle, bone, adipose tissue and the heart.1 Reduced appetite has been proposed as a important factor for weight loss caused by cardiac cachexia.2
The mechanism of cardiac cachexia remains largely unclear. Gastric myoneural inhibition and gastrointestinal hypomotility with delayed gastric emptying is commonly observed, and gut hormones may also play a role, because the anorexia is often characterised by a premature feeling of fullness and loss of hunger.2 The gut hormone ghrelin stimulates hunger, increases food consumption. and was previously shown to be raised in patients with cardiac cachexia and CHF.3 Ghrelin is produced from the stomach and usually rises in anticipation of a meal and then falls after the ingestion of nutrients.
The gut hormone PYY3–36, acting as a terminator of hunger, is produced by the L cells in the distal gastrointestinal tract; it reduces appetite and 24 hour food intake by binding to the neuropeptide Y Y2 receptor in the hypothalamic arcuate nucleus.4
Severe pulmonary hypertension results in a low cardiac output and neurohumoral activation. Patients frequently report loss of appetite and weight, and since it occurs in a young population they have little co-morbid disease, which itself might affect appetite. This study aimed to evaluate dynamic PYY and ghrelin responses to a standard meal in control subjects and patients with cardiac cachexia associated with pulmonary hypertension.
METHODS
Patients with primary pulmonary hypertension and cardiac cachexia, as defined by weight loss of more than 6%,1 underwent diagnostic work up to determine aetiology and severity of pulmonary hypertension according to the World Health Organization and British Cardiac Society criteria. The patients in this study were in WHO functional class III or above, the time since onset of disease was 4.9 (2.1) years, and the shuttle walking test distance was 211.3 (98.5) m. They had a systolic pulmonary artery pressure of 93 (12.1) mm Hg, pulmonary artery oxygen saturation of 52.6 (13.1%), Fick cardiac index of 2.3 (1.5) l/min/m2, and the pulmonary vasculature resistance was 1667 (1103) dynes/cm5/s. Patients involved in the study were taking the following medication: warfarin (nine patients), bosentan (five patients), digoxin (four patients), spironolactone (three patients), treprostinil (three patients), frusemide (two patients) and amlodipine, candesartin, ramipril, montelukast, and iloprost, each being taken by one of the patients. Sodium, creatinine, albumin, and protein were within the reference range in all patients. The study was performed in accordance with the Declaration of Helsinki and informed consent was obtained.
Table 1 demonstrates the demographics of the nine patients and nine control subjects (recruited through local advertising) who received a 720 kcal standard breakfast between 7–8 am following an overnight fast. Blood samples were obtained every 30 minutes starting 30 minutes before the meal and ending 120 minutes after the meal. Samples were immediately centrifuged, plasma separated and stored at −80°C until analysis using established in-house assays.4 Parametric statistical analysis was done using Sigmastat v2.0 (SPSS Science, Chicago, Illinois, USA). The PYY, ghrelin, and body mass index (BMI) data were normally distributed and statistical comparisons were made using an unpaired t test. Linear regression was used to evaluate the correlation between BMI, fasting ghrelin, and peak PYY. A probability level of p ⩽ 0.05 (5%) was considered significant.
Table 1.
Demographics of patients and control subjects
| Cardiac cachexia | Control | p Value | |
| Subjects (female) | 6/9 | 5/9 | 0.7 |
| Age (years) | 38.4 (5.0) | 29.9 (1.6) | 0.13 |
| BMI (kg/m2) | 19.8 (1.3) | 23.6 (0.9) | 0.052 |
| Weight loss (kg) | 9.0 (0.4) | 0.2 (0.1) | <0.001 |
RESULTS
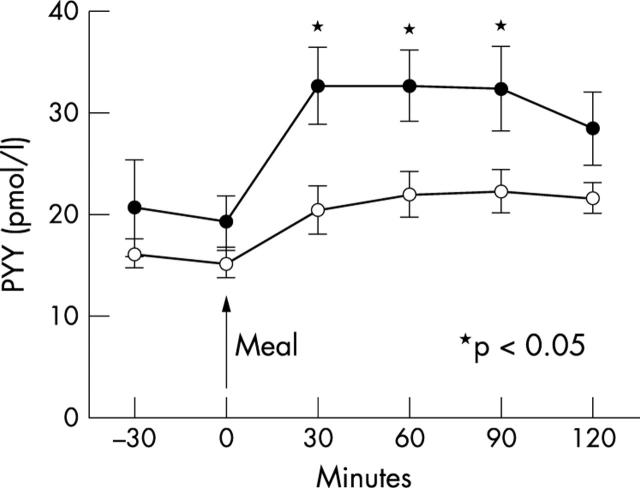
There were no differences in age, sex, and BMI between control subjects and patients (table 1). PYY was 60% higher in the patients post-meal at 30 minutes (p = 0.01), 49% higher at 60 minutes (p = 0.02), and 46% higher at 90 minutes (p = 0.04) when compared with the control subjects (fig 1). There was no difference in fasting PYY concentrations (p = 0.42).
Figure 1.
PYY response following standard meal in cardiac cachexia associated with pulmonary hypertension and control subjects (solid circles, patients with cardiac cachexia; open circles, control subjects)
Both groups showed the expected pattern of reduction in ghrelin post-meal. The fasting ghrelin concentrations for the patients and control subjects were 704.1 (423) pmol/l and 484.6 (204) pmol/l, respectively (p = 0.13), while the 90 minute post-meal values were 470.9 (231) pmol/l and 325.4 (154) pmol/l (p = 0.08).
There was no correlation between BMI and peak PYY response in either the control subjects (p = 0.19) or the cardiac cachexia patients (p = 0.53), and no correlation between BMI and fasting ghrelin for either the control subjects (p = 0.28) or the cardiac cachexia patients (p = 0.53).
DISCUSSION
Patients with cardiac cachexia caused by severe pulmonary hypertension have an exaggerated and early PYY response to a 720 kcal meal when compared to control subjects, while ghrelin showed the same post-meal pattern in both groups.
The pattern of PYY is consistent with previous studies2 and clinical observations which reported that patients with cardiac cachexia experience a change in appetite, possibly reaching satiety earlier and consequently consuming less food per day. A reduction in food intake of as little of 100 kcal per day, if sustained, could lead to weight loss5 similar to that observed in patients with cardiac cachexia associated with pulmonary hypertension.
Previous studies demonstrated fasting concentrations of ghrelin 61% higher in 28 patients with CHF and cardiac cachexia compared to 46 patients with CHF without cachexia.3 We observed fasting ghrelin concentrations 45% higher in the patients with pulmonary hypertension than control subjects (p = 0.13). This is the first study to describe the dynamic pattern of ghrelin following standard meals in these patients, showing similar decreases in ghrelin to control subjects. Raised fasting ghrelin concentrations in cardiac cachexia may reflect weight loss and the negative energy balance.
It is unlikely that PYY and ghrelin are the only factors governing appetite. The mechanism for the elevation of PYY in patients with pulmonary hypertension is not known. It may form part of a metabolic syndrome related to low cardiac output and/or congestion of the gastrointestinal system which leads to the release of these hormones.
In conclusion, the PYY response of patients with cardiac cachexia associated with primary pulmonary hypertension is premature and exaggerated and may play a role in the aetiology of premature hunger termination and reduced daily calorie intake in patients with cardiac cachexia.
Acknowledgments
The authors would like to thank the Wellcome Trust for the Clinical Research Fellowship of CW le Roux and the Medical Research Council for the project grant of SR Bloom.
REFERENCES
- 1.Anker SD, Negassa A, Coats AJ, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 2003;361:1077–83. [DOI] [PubMed] [Google Scholar]
- 2.Pittman J, Cohen P. The pathogenesis of cardiac cachexia. N Engl J Med 1964;271:403–8. [DOI] [PubMed] [Google Scholar]
- 3.Nagaya N, Uematsu M, Kojima M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation 2001;104:2034–8. [DOI] [PubMed] [Google Scholar]
- 4.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 2002;418:650–4. [DOI] [PubMed] [Google Scholar]
- 5.Hill JO, Wyatt HR, Reed GW, et al. Obesity and the environment: where do we go from here? Science 2003;299:853–5. [DOI] [PubMed] [Google Scholar]