Abstract
Objective: To compare characteristics, management, and outcome of myocardial infarction (MI) in men and women.
Design: Prospective observational study.
Setting: District general hospital in southwest Scotland.
Participants: 966 men and 597 women admitted with first MI between 1994 and 2000 with follow up to the end of 2001.
Results: 393 (40.7%) men and 305 (51.1%) women died during a median follow up of 3.4 years for the survivors. Univariate analysis indicated an excess mortality among women (hazard ratio (HR) 1.45, 95% confidence interval (CI) 1.25 to 1.68), which disappeared after adjustment for age, smoking, co-morbidity, previous vascular disease, diabetes, hypertension, and social deprivation (HR 1.02, 95% CI 0.87 to 1.20). There was also an excess early mortality within 30 days among women (HR 1.54, 95% CI 1.20 to 1.98), though this did not retain significance after adjustment for the same covariates (HR 1.04, 95% CI 0.79 to 1.37). Small and insignificant differences were found in the proportion of men and women receiving thrombolysis on admission and secondary prophylactic drugs at discharge, except for statins and β blockers, which were respectively more (adjusted odds ratio 1.48, 95% CI 1.10 to 1.98) and less (adjusted odds ratio 0.78, 95% CI 0.60 to 1.00) commonly prescribed to women.
Conclusion : Results suggest that the poorer outcome for women after MI reported in other studies may reflect sex bias in management as well as differences in age and co-morbidity and support the view that if women have access to the same quality of care as men then survival will be the same.
Keywords: sex, myocardial infarction, outcome
Most but not all studies suggest that women are less likely to develop coronary heart disease than men but are more likely to die when they do.1–6 Many reasons have been advanced to explain this observation. Women tend to be older than men at the time of their myocardial infarction (MI)1–6 and have more co-morbidity.1–6 Women are less likely to be admitted to coronary care units than men.1,6,7 Thrombolysis rates,1,2,4–8 participation in cardiac rehabilitation programmes,9 and the likelihood of further investigation and treatment may all be lower among women.1,2,6 Against this background we conducted a prospective observational study of all patients admitted with their first MI to a district general hospital (Dumfries Infirmary) between the start of 1994 and the end of 2000 with follow up to the end of May 2001. Dumfries is ideal for this type of study: it is the only district general hospital in the region, which has a population of about 150 000, and all patients with suspected MI are admitted to a single coronary care unit irrespective of age or sex.10
PATIENTS AND METHODS
We used the World Health Organization criteria for the diagnosis of MI: any two of typical history, diagnostic ECG changes, or more than twice the upper limit of normal enzyme rise. Thrombolysis was given for all ST elevation MI within 12 hours of the onset of pain provided that no contraindications were present. None of the patients in this study had access to primary angioplasty. Deaths and the date of death were provided by the hospital’s Patient Administration System, which receives mortality statistics from the Registrar General for Scotland. Comparisons between sexes of baseline data taken on or before admission to hospital were by Fisher’s exact test for categorical variables and a two sample t test for age. Survival of men and women was compared unadjusted and then adjusted for age and the other covariates listed in table 1 by Cox’s proportional hazards models (see table 1 footnote for definitions of covariates). All covariates were tested for interactions with sex at the 0.1 level of significance. The outcome of death within 30 days of MI was similarly compared with logistic rather than Cox regression analysis. Kaplan-Meier plots and log rank statistics were used to compare survival of men and women who were younger and older than 75 years. We examined thrombolysis rates on admission for all patients and hospital prescription of secondary prophylactic drug treatment for those who survived to discharge. These data are presented as odds ratios, both unadjusted and adjusted for the same covariates as in the mortality analysis. In addition, we used the Cochran-Armitage one degree of freedom test for trend to examine yearly changes in the prescribing of angiotensin converting enzyme (ACE) inhibitors, statins, aspirin, β blockers, and the use of thrombolytic treatment over the seven year study period.
Table 1.
Descriptive statistics and univariate/multivariate Cox models for time to death
| Factor (level or increment) | Men (n = 966) | Women (n = 597) | Both sexes (n = 1563) | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI)* | p Value |
| Sex (female) | NA | NA | NA | 1.45 (1.25 to 1.68) | <0.0001 | 1.02 (0.87 to 1.20) | 0.81 |
| Age (5 years) | 66.9 (11.8) | 73.2 (11.0) | 69.3 (11.9) | 1.40 (1.34 to 1.45) | <0.0001 | 1.40 (1.34 to 1.46) | <0.0001 |
| Current smoker | 341 (35.5) | 139 (23.5) | 480 (30.9) | 0.49 (0.40 to 0.58) | <0.0001 | 1.00 (0.82 to 1.22) | 0.99 |
| Co-morbidity† | 187 (19.4) | 133 (22.3) | 320 (20.5) | 1.32 (1.11 to 1.57) | 0.0017 | 1.04 (0.87 to 1.25) | 0.66 |
| Previous angina | 258 (26.8) | 157 (26.3) | 415 (26.6) | 1.71 (1.46 to 2.00) | <0.0001 | 1.39 (1.18 to 1.64) | <0.0001 |
| Previous coronary revascularisation | 35 (3.6) | 8 (1.3) | 43 (2.8) | 1.01 (0.64 to 1.59) | 0.98 | 1.55 (0.96 to 2.51) | 0.073 |
| Peripheral vascular disease | 54 (5.6) | 25 (4.2) | 79 (5.1) | 1.39 (1.02 to 1.88) | 0.036 | 1.13 (0.82 to 1.55) | 0.46 |
| Diabetes | 109 (11.4) | 75 (12.7) | 184 (11.9) | 1.78 (1.45 to 2.18) | <0.0001 | 1.70 (1.38 to 2.10) | <0.0001 |
| Hypertension | 227 (23.6) | 190 (32.1) | 417 (26.8) | 1.32 (1.12 to 1.55) | 0.0009 | 1.21 (1.02 to 1.42) | 0.028 |
| Social deprivation‡ | 363 (37.6) | 222 (37.2) | 585 (37.5) | 0.84 (0.72 to 0.98) | 0.024 | 0.87 (0.74 to 1.02) | 0.088 |
Data are mean (SD) for age, number (%) of patients for other categorical factors, and hazard ratio (HR) with 95% confidence interval (CI) and associated p value from univariate/multivariate Cox models for time to death. The prevalence of each covariate was determined at the time of myocardial infarction.
*Data were missing for 25 (1.6%) patients in the multivariate model.
†Arthritis and chronic obstructive airways disease.
‡Carstairs deprivation category ⩾4.
RESULTS
First MI was confirmed prospectively in 966 men and 597 women. During a median follow up of 3.4 years (maximum seven years) for the survivors, 393 (40.7%) men and 305 (51.1%) women died. One hundred and sixty nine (17.5%) of the men and 147 (24.6%) of the women died within 30 days of MI. Table 1 shows baseline data for men and women. Women were older (p < 0.0001) and smoked less (p < 0.0001), and more of them had hypertension (p = 0.0003). Similar proportions of men and women had diabetes (p = 0.42), co-morbid illnesses (p = 0.18), and previous vascular disease (p = 0.55) or were socially deprived (p = 0.87).
Univariate analysis indicated an excess mortality among women (hazard ratio 1.45, 95% confidence interval (CI) 1.25 to 1.68), which disappeared (hazard ratio 1.02, 95% CI 0.87 to 1.20) after adjustment for age, smoking, co-morbidity, previous angina, previous coronary revascularisation, peripheral vascular disease, diabetes, hypertension, and social deprivation. There was also an excess early mortality (within 30 days) among women, though this did not retain significance after adjustment for the same covariates: the odds ratio for women to men was 1.54 (95% CI 1.20 to 1.98, p = 0.0007) in the univariate analysis and 1.04 (95% CI 0.79 to 1.37, p = 0.81) in the multivariate analysis.
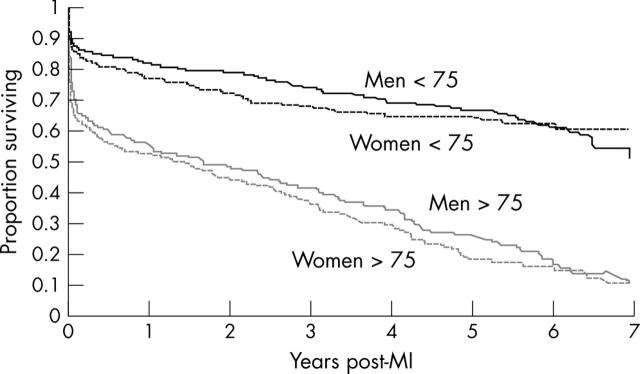
Figure 1 shows the impact of age and sex on survival in men and women who were under and over 75 years at the time of their MI. Five year survival for both sexes combined was 66% for younger and 22% for older patients (p < 0.0001). By contrast the differences in survival between men and women in each age group were small and insignificant (p = 0.31 for patients under 75, p = 0.15 for patients over 75). Increasing age, angina, peripheral vascular disease, and diabetes were associated with adverse outcome in both sexes, though there was no evidence of an interaction between sex and any of the covariates in respect to death.
Figure 1.
Kaplan-Meier curve of survival after myocardial infarction (MI) by age and sex.
We found small and insignificant differences (having adjusted for the stated covariates) in the proportion of men and women receiving thrombolysis on admission (p = 0.52) and an ACE inhibitor (p = 0.18) or aspirin (p = 0.60) at the time of discharge (table 2). Prescription rates were higher for statins among women than men (adjusted odds ratio 1.48, 95% CI 1.10 to 1.98, p = 0.0092), and lower for β blockers (adjusted odds ratio 0.78, 95% CI 0.60 to 1.00, p = 0.051). The use of thrombolysis fell during the seven year study period 1994–2000 for both men and women (combined rate down from 57% to 43%, test for trend p < 0.0001) whereas treatment with all four secondary prophylactic drug rose among both sexes during the course of the study (ACE inhibitors from 44% to 86%, p < 0.0001; statins 3% to 46%, p < 0.0001; β blockers 26% to 60%, p < 0.0001; and aspirin 86% to 91%, p = 0.059). A separate but related analysis of 688 patients who experienced an MI between 1 January 2000 and 31 December 2002 showed that 80% of men and 75% of women who were younger than 70 at the time of their infarct, alive at the time of discharge, and not temporarily resident completed some form of outpatient rehabilitation, either an exercise class or the Heart manual, or both.11
Table 2.
Uptake of thrombolysis and secondary prophylactic drug treatment in men and women
| Treatment | Men (n = 821) | Women (n = 458) | Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value |
| Thrombolysis* | 485 (50.6) | 259 (43.8) | 0.76 (0.62 to 0.93) | 0.009 | 0.93 (0.75 to 1.16) | 0.52 |
| ACE inhibitors | 427 (52.0) | 227 (49.6) | 0.91 (0.72 to 1.14) | 0.40 | 0.85 (0.66 to 1.08) | 0.18 |
| Statins | 196 (23.8) | 109 (23.8) | 1.00 (0.76 to 1.30) | 0.99 | 1.48 (1.10 to 1.98) | 0.0092 |
| Aspirin | 741 (90.3) | 397 (86.7) | 0.70 (0.49 to 1.00) | 0.051 | 0.90 (0.62 to 1.32) | 0.60 |
| β Blockers | 401 (48.8) | 174 (38.0) | 0.64 (0.51 to 0.81) | 0.0002 | 0.78 (0.60 to 1.00) | 0.051 |
Data are number (%) of patients. Odds ratios (OR) are for the ratio of women to men with 95% confidence intervals (CI) and associated p values. Adjustment was for age and for all other covariates in table 1.
*Thrombolysis analysis based on all patients at admission. Prescription of secondary prophylactic drug treatment was for those surviving to hospital discharge.
ACE, angiotensin converting enzyme.
DISCUSSION
There were no differences in survival between men and women after MI after adjustment for age and other confounding variables. A particular strength of our study is that all patients who were admitted to hospital with a diagnosis of MI were included, unlike the reports from some registries in which data for only selected patients were analysed.5 We did not analyse deaths occurring before hospitalisation because we had no access to these data. An analysis from one of the MONICA (monitoring trends and determinants in cardiovascular disease) centres has shown that men are more likely than women to die before admission to hospital and that women are more likely to die after admission. The overall adjusted mortality in this report was the same for both sexes.12 Similar findings have been reported by others.7,13
Our study confirms that female sex is strongly related to factors that confound the relation between sex and risk, and highlights the importance of adjusting mortality for these confounding variables. Our women were older and more likely to be hypertensive than men but less likely to smoke and have undergone coronary revascularisation before their MI. We did not find a difference in the presence of diabetes though others have reported that this tends to be more common among women.2–7 We did not routinely collect data on heart failure but would not have expected a high prevalence among men and women presenting with their first MI. Others have shown that, in addition to being older, women tend to be heavier than men,14 raising the possibility that women are more susceptible to a constellation of risk factors (age, obesity, diabetes, and hypertension) that predict poorer outcomes.
We have previously reported that similar percentages of men and women under 65 years old were admitted to the coronary care unit and received inpatient cardiac rehabilitation, as well as that there were no sex differences in the proportion of these men and women who were still smoking, were taking a lipid lowering drug, had returned to work at one year, or underwent coronary angiography by 3.5 years.10 Our latest findings in a larger population of patients and now including all ages confirm that there are no sex differences in adjusted survival and show that with the exception of statins, which were prescribed more and β blockers less for women, the use of thrombolysis and prophylactic drug treatment, particularly aspirin and ACE inhibitors, is reassuringly similar in both sexes. The fall in thrombolysis rates with time probably reflects the increasing proportion of acute coronary syndromes that are due to non-ST elevation MI and unstable angina, whereas the increasing uptake of secondary prophylactic drug treatment with time is in keeping with current trends.15 Our data on uptake and adherence to cardiac rehabilitation show no important differences between men and women, possibly indicating a proactive approach towards rehabilitating women that has not always been a feature of other programmes.9
In summary, we found no differences in either early or late mortality between men and women after MI, after adjustment for age and other confounding variables. It is tempting to speculate, therefore, that the differences in survival reported in other studies may reflect sex bias in management as well as differences in age and co-morbidity, and to suggest that if women have access to the same quality of care as men their survival will be the same. We believe that the data reported in this and in our earlier study support this view.
Acknowledgments
CI and KH had the idea. KH collected the data and undertook the first analysis. DG was responsible for the literature review and JN for the statistics. CI wrote the first draft. CI, DG, JN all contributed to subsequent drafts and final revision of the paper.
Abbreviations
ACE, angiotensin converting enzyme
CI, confidence interval
MI, myocardial infarction
MONICA, monitoring trends and determinants in cardiovascular disease
REFERENCES
- 1.Mahon NG, McKenna CJ, Codd MB, et al. Gender difference in the management and outcome of acute myocardial infarction in unselected patients in the thrombolytic era. Am J Cardiol 2000;85:921–6. [DOI] [PubMed] [Google Scholar]
- 2.Gan SC, Beaver SK, Houck PM, et al. Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med 2000;343:8–15. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Krumholz HM, Yarzebski J, et al. Sex differences in 2-years mortality after hospital discharge for myocardial infarction. Ann Intern Med 2001;134:173–81. [DOI] [PubMed] [Google Scholar]
- 4.de Gevigney G, Mosnier S, Ecochard R, for the PRIMA group, et al. Are women with acute myocardial infarction managed as well as men? Does it have consequences on in-hospital mortality? Analysis of an unselected cohort of 801 women and 1,718 men. Acta Cardiol 2001;56:169–79. [DOI] [PubMed] [Google Scholar]
- 5.Heer T, Shiele R, Schneider S, et al. Gender differences in acute myocardial infarction in the era of reperfusion (the MITRA registry). Am J Cardiol 2002;89:511–7. [DOI] [PubMed] [Google Scholar]
- 6.Hanratty B, Lawlor DA, Robinson MB, et al. Sex differences in risk factors, treatment and mortality after acute myocardial infarction: an observational study. J Epidemiol Commun Health 2000;54:912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundberg V, Wikstrom B, Bostrom S, et al. Exploring sex differences in case fatality in acute myocardial infarction or coronary death events in the northern Sweden MONICA project. J Intern Med 2002;251:235–44. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan KL, Fitzpatrick P, Cox C, et al. Use of thrombolytic therapy for acute myocardial infarction: effects of gender and age on treatment rates. J Thromb Thrombolysis 2002;13:21–6. [DOI] [PubMed] [Google Scholar]
- 9.Daly J, Sindore AP, Thompson DR, et al. Barriers to participation in and adherence to cardiac rehabilitation programs: a critical literature review. Prog Cardiovasc Nurse 2002;17:8–17. [DOI] [PubMed] [Google Scholar]
- 10.Bannerman A, Hamilton K, Isles C, et al. Myocardial infarction in men and women under 65 years of age: no evidence of gender bias. Scot Med J 2001;46:73–8. [DOI] [PubMed] [Google Scholar]
- 11.McMeeken K, Mark J, McGoey P, et al. Uptake of cardiac rehabilitation is under estimated by failure to include in patient rehabilitation and contribution of the heart manual [abstract]. Eur J Cardiovasc Prevention Rehab 2004;11 (suppl 1) :P–148. [Google Scholar]
- 12.Tunstall-Pedoe H, Morrison C, Woodward M, et al. Sex difference in myocardial infarction and coronary deaths in the Scottish MONICA population of Glasgow 1985–91: presentation, diagnosis, treatment and 28 day case fatality of 3991 events in men and women. Circulation 1996;93:1981–92. [DOI] [PubMed] [Google Scholar]
- 13.MacIntyre K, Stewart S, Capewell S, et al. Gender and survival: a population based study of 201,114 men and women following a first acute myocardial infarction. J Am Coll Cardiol 2001;38:729–35. [DOI] [PubMed] [Google Scholar]
- 14.Montaye M, De Bacquer D, De Backer G, et al. on behalf of the EURO-ASPIRE Investigators. Overweight and obesity: a major challenge for coronary heart disease secondary prevention in clinical practice in Europe. Eur Heart J 2000;21:808–13. [DOI] [PubMed] [Google Scholar]
- 15.Anon. EUROASPIRE I and II Group. Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in 9 countries, Lancet 2001;357:995–1001. [DOI] [PubMed] [Google Scholar]