Sirolimus eluting stents are associated with very low restenosis rates when deployed for single de novo native coronary lesions.1 These and other drug eluting stents are changing the face of interventional cardiology practice.2 A number of fundamental practical questions arise with the use of these novel stents, including the effects on in-stent restenosis (ISR) of overlapping stents, tandem stents, and deploying drug eluting and bare metal stents in the same vessel, as well as the ongoing issue of higher ISR rates in patients with diabetes.1 While rabbit iliac and porcine coronary models of ISR are often used for stent evaluation, these models are expensive and subject to a number of well documented limitations.3 A simple, more cost effective model of ISR would therefore be of major utility. Such a model is described here.
METHODS
All studies were approved by the University of New South Wales ethics committee. Male Sprague-Dawley rats (350–450 mg) were anaesthetised using ketamine and xylazine in the standard manner. A midline incision was made, and access to the left common carotid artery achieved using standard surgical techniques. The vessel was then isolated from the surrounding tissues, and an arteriotomy performed. A 2.0 × 7 mm BiodivYsio human coronary stent (Abbott Vascular, Illinois, USA) was then advanced into the vessel after topical application of 3–5 drops of glyceryl trinitrate (100 μg/ml). No fluoroscopic guidance was used. At a pre-marked point corresponding to the lower thoracic aorta, the stent was deployed using a single inflation at 16 atmospheres for 10 seconds. The stent balloon was then withdrawn, and post-deployment dilatation performed using a 3.0 × 15 mm coronary angioplasty balloon (Boston Scientific, Boston, Massachusetts, USA) advanced in a similar manner, and inflated to 16 atmospheres for 10 seconds. Before the procedure, the shafts of both stent and post-deployment dilation balloon were marked, comparing to surface anatomy. This ensured they both corresponded to the lower thoracic aorta, and that the post-dilation balloon would lie in the centre of the deployed stent.
At selected time points, rats were euthanased with pentobarbitone. The aortas were dissected free, washed briefly in phosphate buffered solution, placed in 10% formalin, and then processed in methylmethacrylate (MMA) polymer. Sections (250 μm) were then cut at 500 μm intervals along the entire stented length using a band cutting system (Exakt GmbH, Norderstedt, Germany), polished, stained with haematoxylin and eosin, and imaged under light microscopy.
All sections along the stented length were examined, and the section exhibiting the greatest degree of ISR formation for each vessel was analysed by computed morphometry. In most cases this was in the central portion of the stented segment. The cross sectional areas for lumen, neointima, and media were measured, and the neointima to media (N/M) ratio derived. Stent strut induced vessel injury was also measured, defined using two previously described injury scores.4,5 A Wilcoxon signed rank test for unpaired data was used to compare groups, with a probability value of p < 0.05 considered significant (table 1).
Table 1.
Histologic evaluation
| 14 Days (n = 3) | 1 Month (n = 9) | 2 Months (n = 6) | |
| Neointima | 0.10 (0.04) | 0.38 (0.10)* | 0.33 (0.08) |
| Media | 0.58 (0.02) | 0.50 (0.17) | 0.68 (0.09) |
| Neointima/media ratio | 0.18 (0.07) | 0.76 (0.17)* | 0.59 (0.20) |
| Schwartz score5 | 1.0 (0.06) | 1.2 (0.1) | 1.3 (0.1) |
| Gunn score4 | 2.1 (0.1) | 2.1 (0.1) | 2.0 (0.2) |
Data presented as mean (SE).
* p<0.05, significant difference compared to 14 days.
RESULTS
Twenty two stents were deployed in 22 rats, and successfully harvested and examined at the following time points: 14 days (n = 3), 1 month (n = 9), 2 months (n = 6). In an additional four animals, one stent had undergone thrombosis, and three stents were poorly deployed making evaluation impractical.
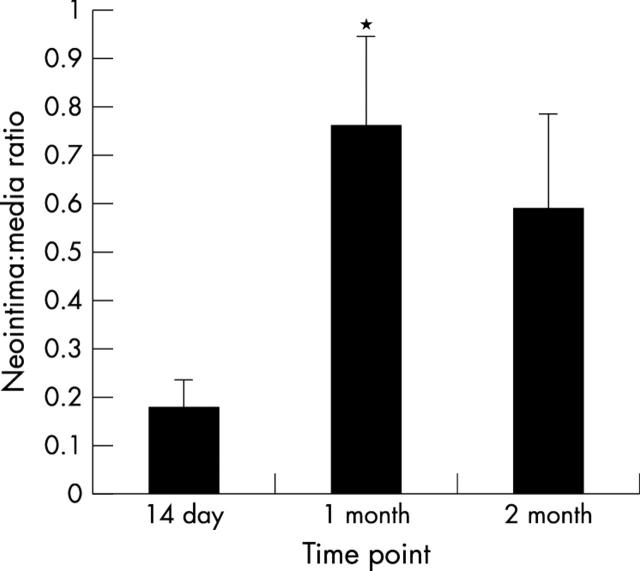
A time dependent concentric area of ISR was observed in all animals (table 1, figs 1 and 2). This was evident by 14 days, though at this early time point, the zone of ISR was largely localised immediately adjacent to the stent struts. At one month, a well defined concentric ring of ISR was present in all animals, with an N/M ratio of 0.76 (0.17). At two months, this was still evident, though there was a trend toward regression in the degree of ISR.
Figure 1.
In-stent restenosis (ISR) following rat aortic stenting. Neointima/media ratios, expressed as mean (SE) at time points of 14 days, one month, and two months. *Significant difference compared to 14 days (p < 0.05, Wilcoxon signed rank test for unpaired data).
Figure 2.
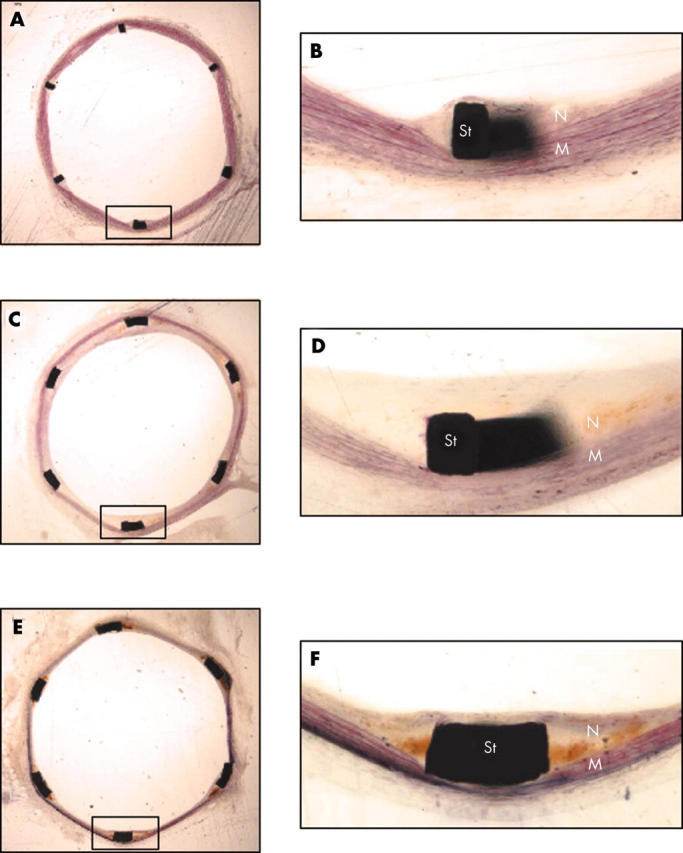
Histology of ISR following rat aortic stenting. (A) section at 14 days; (B) inset from A; (C) section at one month; (D) inset from C; (E) section at two months; (F) inset from E. M, media; N, neointima; St, stent strut.
DISCUSSION
This initial series represents the first description of a model of ISR following overstretch stent injury to the rat aorta. Anecdotal reports have previously suggested that percutaneous stent delivery to the rat aorta is feasible and results in focal, stent strut related ISR at 14 days,6,7 and that following stent placement using an open surgical approach to the lower abdominal aorta, a concentric neointima is present at 56 days.8 This report suggests that the present methodology provides a reliable and reproducible model of ISR.
A number of characteristics of this proposed model are noteworthy. Firstly, that while the absolute area of ISR formation is modest, the N/M ratio is robust, and significant enough to provide a baseline for the evaluation of treatment to reduce ISR. The N/M ratio of 0.76 compares to that of 0.8–2.0 following stenting of the rabbit aorta.9,10 Secondly, this degree of neointimal formation is achieved with only a modest degree of stent strut induced injury. Injury was for the most part created by medial compression without laceration, as reflected in the low mean injury scores (table 1). Based on other animal models, it is likely that a greater degree of injury would result in a more profound degree of neointima,5 perhaps further adding to the utility of this model. Thirdly, the model provides the suggestion of regression of ISR between 1–2 months. Regression of ISR has been observed in humans between six months and three years following stenting11; proposed mechanisms include a maturation of extracellular matrix, with conversion of proteoglycan to elastin,12 and smooth muscle cell apoptosis.13 A greater understanding of the mechanisms of restenosis regression would clearly be of immense clinical value.
Two other rat models of ISR have been recently described, both employing stents deployed on 1.5 mm diameter balloons in the common carotid artery.12,14 The induced ISR is maximal at 21–28 days, with regression at 60 days. However, stenting the rat common carotid artery is technically demanding, and despite a learning curve, these studies were limited by animal mortality of up to 20%, and stent thrombosis rates of 10–26%, which may provide ethical and practical limitations to their widespread use.12,14
The technique described here has some limitations. Firstly, the degree of aortic injury is not precisely quantitated, and the neointima induced is relatively modest. The rat aorta is measured on fixed sections between 2.7–3.0 mm in diameter. Hence a 3.0 mm balloon is dilated to 16 atmospheres to achieve a final balloon to vessel ratio of 1.1–1.2:1. This is not quantified individually. It is possible that direct measurement of the aorta, perhaps using intravascular ultrasound or echocardiography, may allow this. Secondly, present surgical technique does not readily allow the more bulky 3.0 mm pre-mounted stent to be used without frequent trauma to the common carotid artery. The lower profile 2.0 × 7 mm stent avoids this, but requires a second balloon to post-dilate, with the possibility that dilation may not be optimal. Improved stent design and surgical technique may obviate this need.
The cellular composition of the induced neointima using this model is undergoing full evaluation, but at present appears to be formed by smooth muscle cells, with the suggestion of at least some degree of stent strut related thrombus. Investigations are also underway to establish whether more profound degrees of injury are associated with greater degrees of ISR.
Thus, while investigations are ongoing, this report provides the first evidence of reliable and reproducible ISR formation following stenting of the rat aorta. This novel, straightforward model should facilitate rapid and efficient assessment of stent bound local and systemically delivered potential inhibitors of restenosis, and should assist researchers in this rapidly expanding field.
Acknowledgments
HCL is a Clinical Research Fellow of the NHMRC of Australia. The authors thank David Tanous for expert technical assistance.
REFERENCES
- 1.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315–23. [DOI] [PubMed] [Google Scholar]
- 2.Popma JJ, Kuntz RE, Baim DS. A decade of improvement in the clinical outcomes of percutaneous coronary intervention for multivessel disease. Circulation 2002;106:1592–4. [DOI] [PubMed] [Google Scholar]
- 3.Johnson GJ, Griggs TR, Badimon L. The utility of animal models in the preclinical study of interventions to prevent human coronary artery restenosis: on behalf of the subcommittee on animal, cellular and molecular models of thrombosis and haemostasis of the scientific and standardization committee of the international society of thrombosis and haemostasis. Thromb Haemost 1999;81:835–43. [PubMed] [Google Scholar]
- 4.Gunn J, Arnold N, Chan KH, et al. Coronary artery stretch versus deep injury in the development of in-stent neointima. Heart 2002;88:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol 1992;19:267–74. [DOI] [PubMed] [Google Scholar]
- 6.Lowe HC, Chesterman CN, Khachigian LM. Rat aortic stenting: toward a simple model of in-stent restenosis. Am J Cardiol 2001;88:720–1. [DOI] [PubMed] [Google Scholar]
- 7.Lowe HC, Oesterle SN, MacNeill BD, et al. Overstretch stent injury to the rat aorta leads to in-stent restenosis. Am J Cardiol 2002;89:1010. [DOI] [PubMed] [Google Scholar]
- 8.Roks AJ, Henning RH, van Boven AJ, et al. Rat abdominal aortic stenting: a simple model displaying in-stent restenosis. Am J Cardiol 2002;89:1149–50. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzacher SP, Tsao PS, Ward M, et al. Effects of stenting on adjacent vascular distensibility and neointima formation: role of nitric oxide. Vasc Med 2001;6:139–44. [DOI] [PubMed] [Google Scholar]
- 10.Tepe G, Dinkelborg LM, Brehme U, et al. Prophylaxis of restenosis with 186re-labeled stents in a rabbit model. Circulation 2001;104:480–5. [DOI] [PubMed] [Google Scholar]
- 11.Kimura T, Abe K, Shizuta S, et al. Long-term clinical and angiographic follow-up after coronary stent placement in native coronary arteries. Circulation 2002;105:2986–91. [DOI] [PubMed] [Google Scholar]
- 12.Finn AV, Gold HK, Tang A, et al. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J Vasc Res 2002;39:414–25. [DOI] [PubMed] [Google Scholar]
- 13.Bochaton-Piallat ML, Gabbiani F, Redard M, et al. Apoptosis participates in cellularity regulation during rat aortic thickening. Am J Pathol 1996;146:1059–64. [PMC free article] [PubMed] [Google Scholar]
- 14.Indolfi C, Esposito G, Stabile E, et al. A new rat model of small vessel stenting. Bas Res Cardiol 2000;95:179–185. [DOI] [PubMed] [Google Scholar]