Recent prospective clinical trials have demonstrated that physiologic pacing mode reduces the risk of atrial fibrillation (AF) compared to single chamber ventricular pacing in patients with sick sinus syndrome (SSS).1–3 Previous studies have shown that patients with a prior history of AF before pacemaker implant are associated with an increase risk of persistent AF.3 Recent advances in the ability to store diagnostic information in implanted devices have provided an opportunity for detection and documentation of AF. The aim of this study was to define the prevalence and clinical significance of device detected AF episodes in patients implanted with pacemakers for SSS.
METHODS
We studied the clinical characteristics and long term clinical outcome in patients with SSS who had received a dual chamber rate responsive pacemaker (DDDR) for symptomatic bradycardia in our institutions from 1992 to 1998. Patients were eligible if they received a DDDR pacemaker which was capable of providing diagnostic counters for supraventricular tachyarrhythmias and was programmed “ON”. P wave analysis was manually measured by a single investigator. A surface echocardiogram was performed in all patients before implant to assess left atrial size, left ventricular ejection fraction, and left ventricular mass.
Data were retrospectively collected regarding demographic characteristics, device diagnostics, clinical status, and outcome during the follow up period. Clinical data were retrieved from the medical records and subsequently during the most recent clinic visit. The following clinical events of each patient were retrieved from their clinical records: the time to the first occurrence of AF; the development of persistent AF; and major cardiovascular events: non-fatal stroke, hospitalisation for congestive heart failure, or cardiovascular death. The AF episode was categorised as either paroxysmal if sinus rhythm was recorded at the subsequent follow up after an episode of AF, or persistent if it was recorded at two consecutive follow up visits.
Continuous variables are expressed as mean (1 SD). Statistical comparisons were performed with Student’s t test or Fisher’s exact test, as appropriate. Cox regression analysis was used to examine the clinical predictors of major cardiovascular events and persistent AF. Actuarial curves for the incidence of persistent AF and major cardiovascular events in patients with and without device detected AF episodes were calculated by the Kaplan-Meier method. A probability value of p < 0.05 was considered significant.
RESULTS
The clinical characteristics of 226 patients included in this analysis are shown in table 1. After a mean follow up of 84 (16) months, 99 patients (44%) had at least one AF episode documented by the pacemaker. In 30/139 (22%) patients without a prior history of AF, their pacemaker diagnostic counters detected AF after implant. In 18/87 (21%) patients with a prior history of AF, their pacemaker diagnostic counters did not detect any AF after implant. Patients with device detected AF episodes were more likely to have a history of AF before pacemaker implant, underlying structural heart diseases, antiarrhythmic drug treatment and anticoagulation use, larger left atria, lower left ventricular ejection fraction and higher prevalence of left ventricular hypertrophy on echocardiography, and longer P wave duration than patients without AF (table 1).
Table 1.
Clinical characteristics and outcome of patients with or without device detected atrial fibrillation (AF) episodes by pacemaker diagnostic counters after implantation
| AF (n = 99) | No AF (n = 127) | P values* | |
| Age (years) | 72 (10) | 70 (10) | 0.21 |
| Female (%) | 56 | 66 | 0.17 |
| Duration of follow up (months) | 88 (32) | 82(27) | 0.19 |
| Prior history of AF (%) | 55 | 25 | <0.001 |
| Antiarrhythmic | |||
| Baseline (%) | 32 | 5 | <0.001 |
| Follow up (%) | 56 | 6 | <0.001 |
| Anticoagulation | |||
| Baseline (%) | 28 | 3 | <0.001 |
| Follow up (%) | 49 | 6 | <0.001 |
| Underlying heart disease (%) | 76 | 54 | <0.001 |
| Hypertension | 37 | 36 | 0.22 |
| Ischaemic heart disease | 36 | 28 | 0.02 |
| Hypertrophic cardiomyopathy | 9 | 13 | 0.42 |
| Thyroid heart disease | 5 | 5 | 0.66 |
| Dilated cardiomyopathy | 2 | 1 | 0.33 |
| Left atrial diameter (cm) | 4.0 (0.7) | 3.6 (0.7) | 0.02 |
| Left ventricular ejection fraction | 0.50 (0.13) | 0.56 (0.15) | 0.04 |
| Left ventricular mass (g) | 274 (105) | 240 (120) | 0.21 |
| Left ventricular hypertrophy (%) | 39 | 18 | <0.001 |
| P wave duration (ms) | 130 (21) | 122 (17) | 0.049 |
| Combined cardiovascular events (%) | 42 | 15 | <0.001 |
| Non-fatal stroke (%) | 32 | 8 | <0.001 |
| Hospitalisation for congestive heart failure (%) | 15 | 10 | 0.31 |
| Cardiovascular death (%) | 9 | 4 | 0.16 |
*Comparison between the presence and the absence of atrial fibrillation documented by the pacemaker after implantation.
Persistent AF developed in 49/226 (22%) patients. Cox regression analysis demonstrated that a prior history of AF before pacemaker implantation (hazard ratio (HR) 4.2, 95% confidence interval (CI) 2.1 to 10.8; p < 0.001) and the presence of device detected AF episodes (HR 3.1, 95% CI 1.9 to 12.8; p = 0.02) were independent predictors of the development of persistent AF. Kaplan-Meier analysis revealed a significantly higher proportion of persistent AF in patients with a prior history of AF, any device detected AF episodes, or newly device detected AF episodes compared to patients without any AF episodes (p < 0.001) (fig 1).
Figure 1.
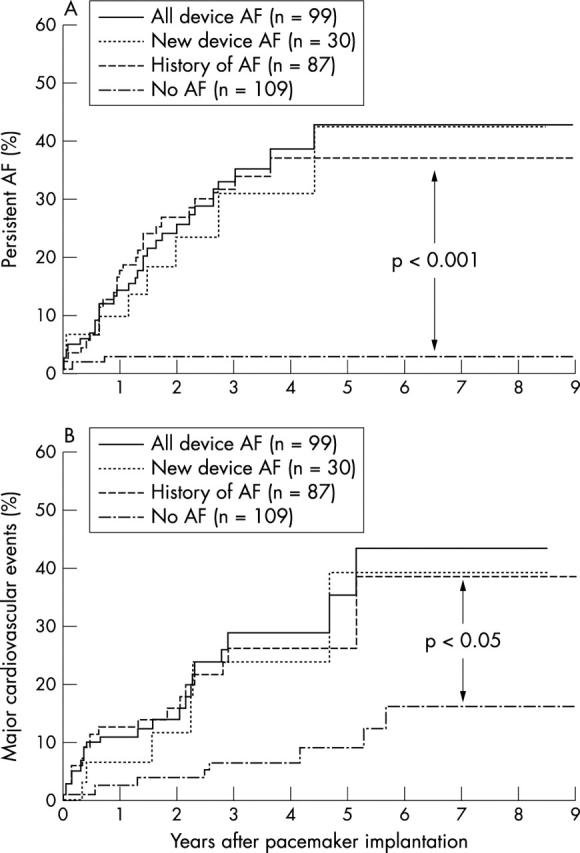
Kaplan-Meier plots depicting the percentage of patients with (A) persistent AF and (B) major cardiovascular events during long term follow up in patients with a prior history of AF (History of AF), any device detected AF episodes (All device AF), newly device detected AF episodes without a prior history of AF (New device AF), and patients without any history or device detected AF episodes (No AF).
Patients with device detected AF episodes had significantly higher prevalence of major cardiovascular events and nonfatal strokes than those without AF episodes (p < 0.001) (table 1). Cox regression analysis showed that a prior history of AF before pacemaker implantation (HR 2.4, 95% CI 1.2 to 13.8; p = 0.013) and the presence of device detected AF episodes (HR 2.0, 95% CI 1.1 to 15.8; p = 0.020) were independent predictors of major cardiovascular events. Kaplan-Meier analysis revealed a significantly higher prevalence of major cardiovascular events in patients with a prior history of AF, any device detected AF episodes, or new device detected AF episodes compared to patients without any AF episodes (p < 0.05) (fig 1).
DISCUSSION
Previous clinical studies have reported that up to two thirds of patients with a pacemaker have atrial arrhythmias detected by the devices.4 However, the clinical significance of this unexpectedly high incidence of non-sustained device detected atrial arrhythmias remains unclear. In this study, we detected AF episodes in 44% of our patients during long term follow up, and it was associated with a 10 fold increase in the incidence of persistent AF, and a 2.5-fold increase in major cardiovascular events, especially stroke. Our results confirm the finding of a recent study,5 and reveal that the presence of device detected AF episodes, irrespective of symptoms, was an independent predictor for persistent AF and major cardiovascular events. Most importantly, our data demonstrated the presence of new device detected AF episodes in 22% patients without a prior history of AF. The occurrence of AF in those patients was also associated with a higher incidence of developing persistent AF and major cardiovascular events during long term follow up. The use of device detected AF episodes defines a subgroup of patients who did not have a prior history of AF at higher risk of developing persistent AF and major cardiovascular events. Therefore, it is clinically valuable to detect AF episodes in those patients so that anticoagulation treatment can be initiated to prevent stroke.
In conclusion, in patients with SSS, AF frequently develops after DDDR pacemaker implantation, and progresses to persistent AF over long term follow up. These data suggest the use of a pacemaker diagnostic counter to detect AF episodes in pacemaker patients, especially in those without a prior history of AF, can assist in identifying patients at risk of developing persistent AF and major cardiovascular events, especially stroke. Anticoagulation treatment for stroke prevention should be considered in those patients who have a prior history of AF or device detected AF episodes.
Abbreviations
AF, atrial fibrillation
CI, confidence interval
HR, hazard ratio
SSS, sick sinus syndrome
This study was not supported by any funding and no author has a real or perceived conflict of interest.
REFERENCES
- 1.Andersen HR, Thuesen L, Baggar JP, et al. Prospective randomized trial of atrial versus ventricular pacing in sick-sinus syndrome. Lancet 1994;344:1523–8. [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346:1854–62. [DOI] [PubMed] [Google Scholar]
- 3.Skanes AC, Krahn AD, Yee R, et al. Progression to chronic atrial fibrillation after pacing: the Canadian trial of physiologic pacing. CTOPP investigators. J Am Coll Cardiol 2001;38:167–72. [DOI] [PubMed] [Google Scholar]
- 4.DeFaye P, Dournaux F, Mouton E. Prevalence of supraventricular arrhythmias from the automated analysis of data stored in the DDD pacemakers of 617 patients: the AIDA study. Pacing Clin Electrophysiol 1998;21:250–5. [DOI] [PubMed] [Google Scholar]
- 5.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the atrial diagnostics ancillary study of the mode selection trial (MOST). Circulation 2003;107:1614–9. [DOI] [PubMed] [Google Scholar]


