Chagas’ disease, caused by the protozoan Trypanosoma cruzi, remains a leading cause of heart disease in Latin America. It is believed that approximately 20 million people are infected with this parasite. An acute phase follows the parasite infection and is characterised by an active infection, inflammation, and myocardial damage.1 Symptoms of chronic cardiomyopathy develop in 20–30% of previously asymptomatic individuals decades after initial infection. Severe congestive heart failure (CHF) is a common finding, and necropsies show notable dilation of all four cardiac chambers. Areas of extensive myocardial fibrosis and left ventricular apical aneurysms are common findings.1
Although atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are predictive of left ventricular hypertrophy and dysfunction, they respond differently, for example, to cardiac volume load in fetal circulation or diabetic cardiomyopathy.2 BNP has recently gained increased importance in the clinical diagnosis of cardiovascular diseases, and BNP guided treatment of CHF was found to reduce cardiovascular morbidity.3
The major studies and clinical trials on CHF have excluded Chagas patients. Thus the aim of our study was: firstly, to describe plasma ANP and BNP concentrations in Chagas patients compared to controls as a screening test for ventricular dysfunction; secondly, to investigate a possible correlation with the functional New York Heart Association (NYHA) class and left ventricular ejection fraction (LVEF) and the prognostic potency in Chagas’ disease; and thirdly, to compare peptide concentrations with those in patients with other dilated cardiomyopathies (DCM) in order to investigate this tool as a diagnostic marker for discriminating between the two types of heart failure.
METHODS
An institutional review committee approved the study, and all patients gave written consent. The study population was examined between July 2001 and February 2003.
Ninety consecutively recruited patients with at least two positive serologies for Chagas’ disease, termed the Chagas group, and 47 patients with negative serology for Chagas’ disease, termed the idiopathic DCM group, were studied and compared with 31 sex and age matched healthy subjects. Table 1 shows the clinical and echocardiographic characteristics of the individuals. Other structural cardiac diseases and co-morbidities were excluded in both groups. All patients received medical treatment according to NYHA functional class. The control group (n = 31) were healthy subjects not taking any medication.
Table 1.
Clinical data of the patient groups
| Groups | Control (n = 31) | CD | DCM | |||
| 0 (n = 43) | NYHA I–II (n = 20) | NYHA III–IV (n = 27) | NYHA I–II (n = 22) | NYHA III–IV (n = 25) | ||
| Age (years) | 54.3 (2.3) | 52.0 (1.5) | 53.2 (3.1) | 48.3 (2.1) | 47.0 (3.1) | 46.9 (2.5) |
| Male/Female | 16/15 | 11/32 | 7/13 | 15/12 | 13/9 | 16/9 |
| SBP (mm Hg) | 120.8 (2.0) | 123.7 (1.8) | 110.6 (2.4)* | 97.7 (2.7)* | 113.7 (2.6)* | 100.8 (3.2)* |
| DBP (mm Hg) | 76.3 (0.9) | 78.1 (1.0) | 74.4 (1.3) | 70.6 (1.5)* | 74.8 (1.4) | 72.0 (1.6)* |
| Heart rate (bpm) | 74.7 (1.1) | 70.1 (1.3) | 71.4 (2.2) | 73.5 (2.3) | 73.4 (2.2) | 81.7 (2.4)* |
| ECG: pacemaker | 4 | 4 | 5 | 0 | 0 | |
| Normal/Abnormal | 16/23 | 0/16 | 0/22 | 0/22 | 0/25 | |
| Echo: LVEF (%) | 68.1 (1.2) | 38.3 (2.0)* | 26.0 (1.5)* | 36.3 (2.3)* | 23.7 (1.4)* | |
| LVEF>50%/<50% | 43/0 | 2/18 | 0/27 | 1/21 | 0/25 | |
*Denotes p<0.05 v Chagas group 0.
CD, Chagas’ disease; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
Abnormal ECG includes arrhythmias, flutter and atrial fibrillation, any bundle branch block, atrioventricular block, atrial/ventricular hypertrophy.
The study patients were prospectively defined and subdivided into five groups: group 1 (n = 43) Chagas’ disease without systolic ventricular dysfunction (LVEF > 50%); group 2 (n = 20) Chagas’ disease with ventricular systolic dysfunction (LVEF < 50%), in NYHA classes I–II; group 3 (n = 27) Chagas’ disease with ventricular systolic dysfunction (LVEF < 50%), in NYHA classes III–IV; group 4 (n = 22) DCM with ventricular systolic dysfunction (LVEF < 50%), in NYHA classes I–II; and group 5 (n = 25) DCM with ventricular systolic dysfunction (LVEF < 50%), in NYHA classes III–IV.
Plasma ANP and BNP concentrations were determined in duplicate by highly sensitive and specific immunoradiometric assays (IRMA: Schering-Berlin, Germany) as described before for BNP.4 All data were expressed as mean and standard error of the mean (SEM). A probability value of p < 0.05 was defined as significant.
RESULTS
Baseline characteristics of patients and controls according to the NYHA functional class are given in table 1. The table also illustrates increased impairment of cardiac function in Chagas’ disease and DCM patients with increasing NYHA class. This impairment was in parallel with increasing medications in both disease collectives (data not shown). As demonstrated for LVEF in table 1, other echocardiographic parameters such as LV end diastolic volume or LV systolic diameter did not significantly differ in the two disease groups (data not shown).
The control patients presented with normal and homogeneous ANP and BNP concentrations. In both disease groups, the ANP was less pronounced than the BNP increase, and plasma concentrations were directly related to NYHA class in both Chagas’ disease and DCM patients (fig 1A). However, BNP, but not ANP, was significantly higher in Chagas patients of NYHA classes I–II than in DCM patients of the same NYHA classes (p < 0.05). Chagas patients without systolic ventricular dysfunction have already been characterised by elevated natriuretic peptide concentrations (for both p < 0.01, fig 1A).
Figure 1.
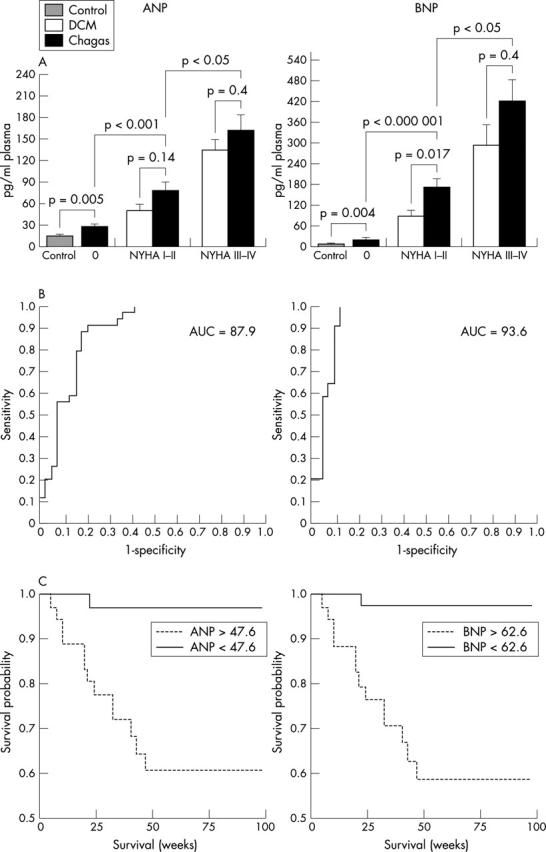
(A) Plasma concentrations of ANP in controls (n = 30), in patients with DCM group divided in NYHA classes I–II (n = 22) and NYHA classes III–IV (n = 22) classification, and in patients with Chagas’ disease (Chagas) distributed in asymptomatic (0) (n = 42), NYHA classes I–II (n = 20), and NYHA classes III–IV (n = 26). Data given as mean (SEM). (B) Receiver operating characteristic (ROC) curves for ability of ANP and BNP to detect left ventricular systolic dysfunction in the whole Chagas’ disease population (LVEF < 50%, n = 45; LVEF > 50%, n = 45). (C) Kaplan-Meier analyses showing cumulative rates of survival in 87 patients with Chagas’ disease according to ANP (A, cut off 47.6) and BNP concentrations (B, cut off 62.6); p = 0.0002 for ANP and p = 0.0001 for BNP.
The Mann-Whitney U test and Pearson’s correlation coefficients were used to analyse the correlation between both the considered peptides in the Chagas patient group. The data demonstrate the strong non-linear relation between both peptides and LVEF, the peptide concentrations increasing exponentially when LVEF decreases (ANP: r = 0.683, p < 10−12; BNP: r = 0.715, p < 10−13). Moreover, all the monitored cardiac parameters recorded by echocardiography evidenced a high correlation with both circulating peptide concentrations, but LVEF reached the highest correlation (data not shown).
The ability of ANP and BNP in discriminating between Chagas patients with LVEF less or greater then 50% was described by receiver operator characteristics curves and it was discovered that already low concentrations of both ANP (47.6 pg/ml, 0.86 sensitivity, 0.79 specificity) and BNP (62.6 pg/ml, 0.95 sensitivity, 0.84 specificity) are able to distinguish between these two subgroups (fig 1B).
There were 10 deaths from pump failure (NYHA class IV), one sudden death (NYHA class II), and four heart transplantations (NYHA class IV) in the Chagas group. In order to find out whether the determined cut off points for ANP and BNP also have predictive value for the lethality of patients with Chagas’ disease, Kaplan-Meier curves were generated using these peptide concentrations to divide the Chagas patients into two subgroups (fig 1C). Classical statistics visualised the significant predictive value of ANP and BNP plasma concentrations for increased risk in lethality or the necessity for heart transplantation (log rank, ANP: p = 0.0002; BNP: p = 0.0001).
DISCUSSION
Our study is the first to investigate both natriuretic peptides in the plasma of Chagas patients. As previously described for DCM, we observed a notable elevation of plasma ANP and BNP concentrations depending on the NYHA class. Since the increase in ANP and BNP in the Chagas group classes III–IV was comparable to that observed in the corresponding DCM, the impaired cardiac function triggers these peptides regardless of the causes that initiate the compensatory mechanisms in advanced heart failure. Surprisingly, BNP concentrations were significantly higher in Chagas than in DCM patients of NYHA class I–II. One explanation may be that most of the DCM patients were on β blockers (20 DCM patients v one Chagas patient). Moreover, recent papers have indicated that elevated BNP concentrations in the heart are associated with cardiac fibrosis. The hypothesis that BNP is triggered more strongly by fibrosis than by blood pressure elevation is also supported by findings from our group.5 Since Chagas’ disease is one of the most fibrogenic cardiomyopathies,1 fibrosis is very prominent and could explain the more pronounced BNP concentrations in the Chagas than in the DCM group.
Our data demonstrates that beside BNP, ANP may also be a strong prognostic marker in chagasic patients. However, for all investigated parameters, BNP was more powerful than ANP; since the differences were mainly marginal, targeted treatment by monitoring circulating peptide concentrations of both ANP and BNP could be helpful for optimising treatment.
The fact that Chagas patients without cardiac dysfunction had already significantly elevated natriuretic peptide concentrations may indicate direct control of the parasite over ANP and BNP expression, or more likely, an early cardiac pathology, and may thus show that natriuretic peptide concentrations could already be used as a marker of asymptomatic Chagas’ disease without ventricular dysfunction. Thus, since early screening of ventricular dysfunction is very important in a population at high risk for heart failure like asymptomatic Chagas’ disease patients, it would be useful to periodically measure natriuretic peptides in this population to screen for incipient ventricular dysfunction.
Acknowledgments
This work was supported by the Humboldt Foundation (SHW) and a grant to TW from the Deutsche Forschungsgemeinschaft (WA-1441/1). The technical assistance of Helmut Würdemann is gratefully acknowledged.
Abbreviations
ANP, atrial natriuretic peptide
BNP, brain natriuretic peptide
CHF, congestive heart failure
DCM, dilated cardiomyopathy
LVEF, left ventricular ejection fraction
NYHA, New York Heart association
REFERENCES
- 1.Higuchi ML, Benvenuti LA, Reis MM, et al. Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovasc Res 2003;60:96–107. [DOI] [PubMed] [Google Scholar]
- 2.Walther T, Stepan H, Faber R. Dual natriuretic response to volume load in the fetal circulation. Cardiovas Res 2001;49:817–9. [DOI] [PubMed] [Google Scholar]
- 3.Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 2000;355:1126–30. [DOI] [PubMed] [Google Scholar]
- 4.Walther T, Heringer-Walther S, Wessel N, et al. Brain natriuretic peptide in Chagas’ disease. Lancet 2003;360:1567. [DOI] [PubMed] [Google Scholar]
- 5.Walther T, Klostermann K, Heringer-Walther S, et al. Fibrosis rather than blood pressure determines cardiac BNP expression in mice. Regulatory Peptides 2003;116:95–100. [DOI] [PubMed] [Google Scholar]


