Abstract
Objective: To investigate the association between Chlamydia pneumoniae and matrix metalloproteinase-9 (MMP-9) in atherosclerotic plaques.
Design: 31 coronary atherosclerotic plaque specimens were studied by immunohistochemistry, polymerase chain reaction (PCR), and reverse transcription PCR for the presence of C pneumoniae antigen and genomic DNA, and of MMP-9 protein and transcripts.
Results: Immunohistochemical analysis identified a strong association between the presence of C pneumoniae antigen and production of MMP-9 in coronary atherosclerotic plaques (p = 0.001). Furthermore, analysis of the intralesional amount of C pneumoniae and MMP-9 indicated an increased number of cells positive for MMP-9 in arterial sections that had increased C pneumoniae positivity (p < 0.05).
Conclusions: This study provides evidence of an association between expression of MMP-9 and the intravascular presence of C pneumoniae and may suggest a potential pathological mechanism whereby C pneumoniae may contribute to the progression of coronary atherosclerosis.
Keywords: atherosclerosis, Chlamydia pneumoniae, immunohistochemistry, matrix metalloproteinase, polymerase chain reaction
Atherosclerosis is now generally considered to be an inflammatory disease, but the underlying mechanisms of induction and progression of the lesions are still unclear.1 The role of infectious agents in atherogenesis has been studied extensively with Chlamydia pneumoniae evoking particular interest in recent years.2
A relation between C pneumoniae and atherosclerosis has been postulated based mainly on the presence of the organism in plaques, experimental studies in animals,3–5 and results of pilot antibiotic trials in patients.6,7,8,9,10,11 Opinion, however, is divided as to the role of C pneumoniae in the pathogenesis of atherosclerosis, as recent studies have yielded controversial results.12,13
Despite the dichotomy of opinion concerning C pneumoniae and atherosclerosis, it is generally accepted that chronic infection of the vascular wall may initiate or exacerbate an ongoing inflammatory response.1 A key component of inflammation is the macrophage population. Macrophages infiltrate early atherosclerotic lesions accumulating oxidised low density lipoprotein. C pneumoniae has been identified within macrophages and smooth muscle cells of atherosclerotic tissue14 and shown to be able to infect and survive in human macrophages, arterial smooth muscle cells, and endothelial cells.14,15 In addition, C pneumoniae induces foam cell formation by macrophages.15,16
Matrix metalloproteinases (MMPs) are proteolytic enzymes that are involved in the degradation of matrix components. The 92 kDa gelatinase MMP-9 is often expressed in atherosclerotic plaques by macrophages and smooth muscle cells17 and degrades the matrix components in the fibrous cap.18 Plaque shoulders, regions of foam cell accumulation, and microvasculature have increased expression of MMP-9.19 It is suggested that MMP-9 has a role in unstable angina17 and vascular remodelling.18 Increased expression of MMP-9 has been associated with the formation of intimal hyperplasia and carotid artery remodelling in a mouse blood flow cessation model.20C pneumoniae and its heat shock protein-60 have been shown to induce expression of MMP-9 and tumour necrosis factor α in mouse macrophages in vitro21 and more recently in human monocyte derived macrophages in vitro.22
The objective of the present study was to investigate the association between intralesional C pneumoniae infection and MMP-9 production. In addition the extent of macrophage infiltration and foam cell accumulation were examined and correlated with cellular localisation of both C pneumoniae and MMP-9.
METHODS
Patients and samples
Human coronary endarterectomy specimens were obtained from 24 patients (21 men, mean age 63 (8) years) requiring elective urgent coronary artery bypass surgery. A further seven human coronary artery samples were obtained from the explanted hearts of patients undergoing cardiac transplantation (seven men, mean (SD) age 44 (11) years). Venous samples from restenotic bypass grafts were excluded. Samples were stored at 4°C until being processed on the same day as surgery. The study protocol was approved by the local ethics committee and all participants gave written informed consent.
Histology and immunohistochemistry
Serial 5 μm cryostat sections of atherosclerotic coronary arteries were analysed by immunofluorescence double labelling. Sections were cut, air dried on multispot slides, and frozen until use. For immunohistochemical analysis, sequential sections were double stained for C pneumoniae and CD68, MMP-9 and CD68, negative control and α smooth muscle cell actin, or CD31 and negative control. Sections were initially blocked with rabbit serum (Dako) containing four drops/ml avidin solution (avidin/biotin blocking kit, Vector Labs) for 15 minutes, washed briefly with phosphate buffered saline, and incubated with the primary antibodies. The primary antibodies, prepared in phosphate buffered saline containing four drops/ml biotin (Avidin/Biotin blocking kit, Vector Labs), were mouse anti-C pneumoniae major outer membrane protein (clone RR402, Dako), mouse anti-MMP-9 (clone 56–2A4, Oncogene Biosciences), or mouse IgG3 (Sigma) used as a negative control. After an overnight incubation at 4°C, the sections were washed and incubated with biotin conjugated rabbit anti-mouse immunoglobulins (Dako) followed by streptavidin/fluorescein isothiocyanate (Dako).
Sections were then labelled with antibodies to the macrophage marker CD68 (Dako), α smooth muscle cell actin (Sigma), or endothelial cell CD31 (Dako) for one hour at room temperature, followed by incubation with biotin conjugated rabbit anti-mouse immunoglobulins (Dako) and streptavidin/7-amino-4-methylcoumarin-3-acetate (Jackson Labs). Immunostaining with the anti-C pneumoniae, anti-MMP-9, and anti-CD68 and the presence of foam cells were assessed by using the following grades: 1+, 2+, 3+, and 4+, corresponding to immunoreactivity in 1–10, 11–20, 20–30, and over 30 cells per × 400 microscope field, and 0 for negative sections. To determine the stage of atherosclerosis, sections were analysed by haematoxylin and eosin staining and the plaques were classified according to the recommendation published by Stary and colleagues.23
To validate the findings and rule out the possibility of false positive specimens caused by artefactual staining, 18 of 22 C pneumoniae positive specimens were re-examined for chlamydial antigen with the mouse C pneumoniae monoclonal antibody (RR402) and a further mouse C pneumoniae monoclonal antibody (clone A57-B9, Affinity Bioreagents Inc) specific for the 60 kD heat shock protein of C pneumoniae. Positive control slides of C pneumoniae infected Hep-2 cells (Dako) were also analysed.
Detection of C pneumoniae DNA by polymerase chain reaction
C pneumoniae genomic DNA was detected by nested polymerase chain reaction (PCR). Briefly, 5 mm long artery sections were digested for three hours at 55°C in 5% sodium dodecyl sulfate and 400 μg/ml proteinase K in tris-EDTA buffer pH 8.0 followed by phenol chloroform DNA extraction and ethanol precipitation. After DNA quantification, 500 μg of DNA was subjected to nested PCR with oligonucleotide primers specific for the major outer membrane protein of C pneumoniae (first round: sense, 5′-TTACAAGCCTTGCTTGTAGG-3′; antisense, 5′-GCGATCCCAAATGTTTAAGGC. Second round: sense, 5′-TTATTAATTGATGGTACAATA-3′; antisense, 5′-ATCTACGGCAGTAGTATAGTT-3′) according to procedures described elsewhere.24 The resulting 207 base pair amplicons were analysed by agarose gel electrophoresis. Amplification of the housekeeping gene myoglobin served as a control confirming the integrity of the extracted DNA.25
Detection of MMP-9 transcript by reverse transcription PCR
MMP-9 transcription was analysed by reverse transcription (RT) -PCR as described.22 Briefly, total RNA was isolated from 5 mm long artery sections with ultrapure TRIzol (Gibco) and reverse transcribed into cDNA with the Superscript II preamplification kit (Gibco). The cDNA was subjected to PCR with oligonucleotide primers specific for human MMP-9 (sense, 5′-CACTGTCCACCCCTCAGAGC-3′; antisense, 5′-GCCACTTGTCGGCGATAAGG-3′).26 To confirm the integrity of the extracted RNA, transcription of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase was analysed as a control (sense, 5′-ACCACAGTCCATGCCATCAC-3′; antisense, 5′-TCCACCACCCTGTTGCTGTA-3′).22 The resulting PCR amplicons were analysed by agarose gel electrophoresis.
Statistical analysis
Statistical analysis was carried out with the SPSS version 10 statistics package (SPSS Inc, Chicago, Illinois, USA). Results were analysed as categorical data with the χ2 test and Fisher’s exact test where necessary. A probability value of p < 0.05 was considered significant.
RESULTS
Immunohistochemical analysis of human coronary artery specimens
Analysis of human coronary atherosclerotic lesions by immunohistochemical double staining showed an association between intralesional presence of C pneumoniae and increased expression of MMP-9. Immunoreactive chlamydial antigen was detected in 22 of 31 specimens (71%) and immunoreactive MMP-9 was detected in 27 of 31 specimens (86%) (table 1). The positive chlamydial staining was re-examined and confirmed in 18 available specimens with the C pneumoniae antibody and a second monoclonal antibody to a distinct chlamydial antigen (fig 1). Incubation of specimens with negative control mouse antibodies resulted in no staining. The four samples that were negative for MMP-9 were also negative for C pneumoniae (table 1). These data support that detection of MMP-9 in coronary atherosclerotic plaques is associated with the intralesional presence of C pneumoniae (p = 0.001). Furthermore, analysis of the intralesional amount of C pneumoniae positivity and MMP-9 production by the grading system outlined above indicated that the number of cells positive for MMP-9 was apparently increased in arterial sections that had a higher level of C pneumoniae positivity. Analysis of these data showed that there was a significant association between the extent of chlamydial infection and the amount of MMP-9 production (p < 0.05).
Table 1.
Summary of immunohistochemical analysis, PCR, RT-PCR, and plaque grade data
| Patient | Age (years)/sex | Macrophage grade | Foam cell grade | C pneumoniae PCR | MMP-9 RT-PCR | Stage of atherosclerosis* | Immunostaining | |
| C pneumoniae | MMP-9 | |||||||
| 1 | 58/M | 4+ | 1+ | – | + | VIa | 3+ | 2+ |
| 2 | 67/M | 1+ | 0 | + | + | VIc | 1+ | 1+ |
| 3 | 63/M | 2+ | 2+ | – | + | IV | 4+ | 2+ |
| 4 | 59/M | 2+ | 0 | – | + | Vc | 1+ | 1+ |
| 5 | 51/M | 3+ | 1+ | – | + | Vc | 2+ | 2+ |
| 6 | 53/M | 3+ | 2+ | – | + | Vb | 0 | 3+ |
| 7 | 70/F | 4+ | 3+ | – | + | Va | 3+ | 3+ |
| 8 | 37/M | 1+ | 0 | – | + | II | 4+ | 4+ |
| 9 | 66/M | 2+ | 1+ | – | + | Vb | 3+ | 2+ |
| 10 | 49/M | 2+ | 3+ | – | + | Vb | 0 | 2+ |
| 11 | 60/M | 2+ | 3+ | – | + | Vb | 1+ | 1+ |
| 12 | 69/M | 2+ | 3+ | – | – | Vb | 0 | 0 |
| 13 | 52/M | 4+ | 3+ | – | + | Vc | 0 | 1+ |
| 14 | 58/M | 3+ | NT | – | + | IV | 2+ | 3+ |
| 15 | 35/M | 1+ | NT | – | + | I | 0 | 2+ |
| 16 | 27/M | 2+ | 2+ | + | + | Va | 2+ | 2+ |
| 17 | 48/M | 1+ | 0 | – | – | Vb | 0 | 0 |
| 18 | 56/F | 4+ | 4+ | – | NT | VIa | 0 | 2+ |
| 19 | 55/M | 3+ | 1+ | – | + | Va | 3+ | 1+ |
| 20 | 53/M | 2+ | 2+ | – | NT | IV | 3+ | 2+ |
| 21 | 75/M | 1+ | 1+ | – | NT | Vc | 2+ | 2+ |
| 22 | 68/M | 3+ | 2+ | – | + | Va | 2+ | 3+ |
| 23 | 71/M | 2+ | 0 | + | + | Va | 1+ | 2+ |
| 24 | 65/M | 2+ | 1+ | – | – | III | 3+ | 1+ |
| 25 | 70/M | 2+ | 0 | – | + | Vb | 2+ | 1+ |
| 26 | 72/M | 3+ | 2+ | – | + | Vc | 2+ | 2+ |
| 27 | 50/M | 3+ | 3+ | – | + | Vb | 4+ | 2+ |
| 28 | 59/F | 1+ | 0 | – | – | Va | 0 | 0 |
| 29 | 69/M | 2+ | 1+ | + | + | Va | 2+ | 1+ |
| 30 | 76/M | 3+ | 1+ | – | + | Vc | 2+ | 1+ |
| 31 | 64/M | 2+ | 3+ | – | – | Vc | 0 | 0 |
Immunohistochemistry data shown as grades 0 to 4+.
*Classified according to the recommendations by Stary et al.25
C pneumoniae, Chlamydia pneumoniae; F, female; M, male; MMP-9, matrix metalloproteinase-9; NT, not tested; PCR, polymerase chain reaction; RT-PCR, reverse transcription polymerase chain reaction.
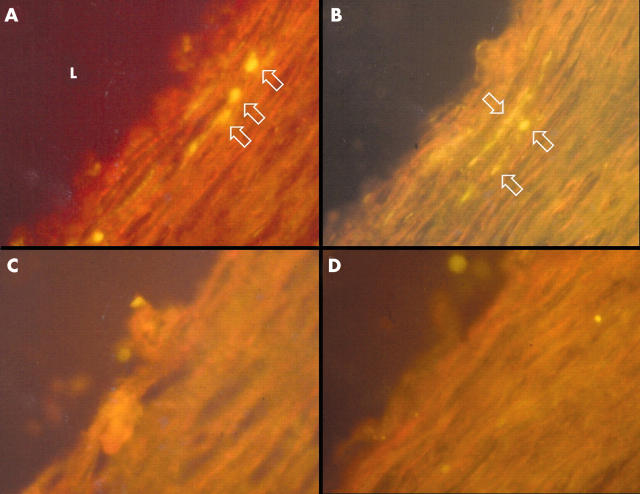
Figure 1.
Immunohistochemical staining of 5 μm sections of a coronary endarterectomy specimen with monoclonal antibodies to two distinct antigens of Chlamydia pneumoniae. Sequential sections stained with (A) fluorescein isothiocyanate (FITC) labelled C pneumoniae major outer membrane protein antibody (clone RR402) and (C) an IgG3 isotype control antibody. Sequential sections of the same specimen stained with (B) FITC labelled C pneumoniae heat shock protein-60 antibody (clone A57-B9) and (D) an IgG1 isotype control antibody. Arrows indicate positively stained cells. L, artery lumen. (Original magnification ×400).
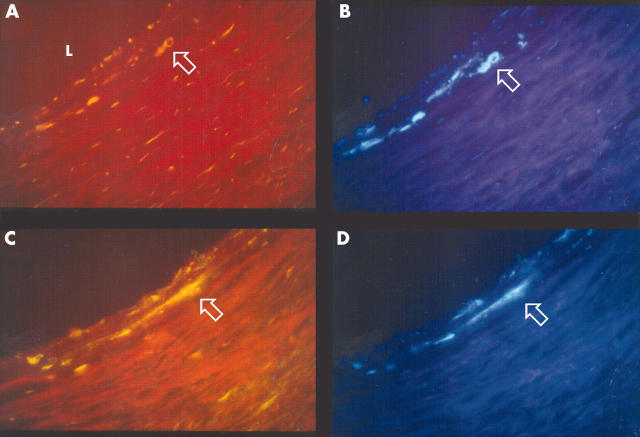
To determine the cellular localisation of C pneumoniae and MMP-9, double immunostaining was carried out with C pneumoniae antibody or anti-MMP-9 antibody with CD68 antibody, and sequential sections were immunostained with α smooth muscle cell actin and anti-CD31 antibodies. Chlamydial antigen co-localised with macrophages (fig 1), smooth muscle cells, and endothelial cells. MMP-9 was observed in macrophages, smooth muscle cells, and endothelial cells (figs 2 and 3). Both MMP-9 and C pneumoniae were often observed within macrophage rich regions in the plaque (fig 2) and in areas of extensive smooth muscle cell infiltration such as the shoulder regions of the plaque. Analysis of serial sections showed that MMP-9 co-localised with C pneumoniae in 13 of 19 specimens (68%).
Figure 2.
Double immunohistochemical staining of 5 μm sections of a coronary endarterectomy specimen showing co-localisation of matrix metalloproteinase-9 (MMP-9) and C pneumoniae in the arterial wall. Sequential sections stained with FITC labelled antibodies to (A) C pneumoniae (RR402) and (C) MMP-9. (B, D) The same sections stained with 7-amino-4-methylcoumarin-3-acetate (AMCA) labelled CD68 antibody. Arrows indicate positively stained cells. (Original magnification ×400).
Figure 3.
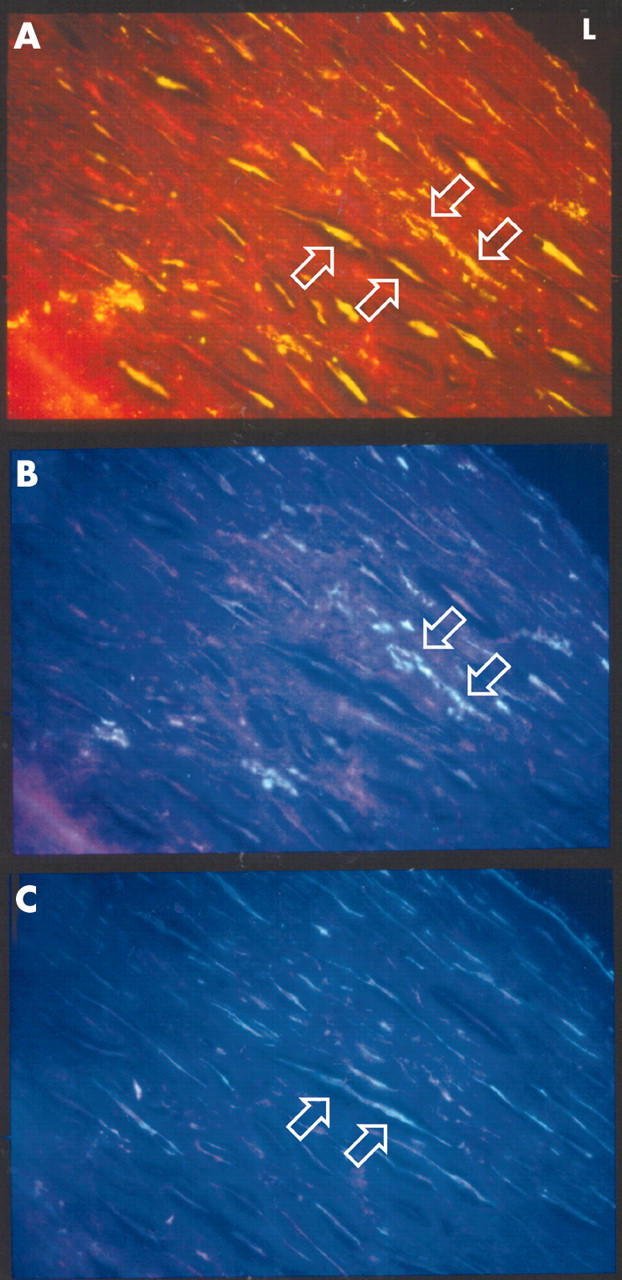
Double immunohistochemical staining of 5 μm sections of a coronary endarterectomy specimen showing smooth muscle cells and macrophages expressing MMP-9. (A) FITC staining of MMP-9. (B) AMCA staining of CD68. (C) AMCA staining of α smooth muscle cell actin. Upward arrows indicate cells positive for α smooth muscle cell actin and MMP-9 stain. Downward arrows indicate cells positive for CD68 and MMP-9 stain. (Original magnification ×400).
Immunohistochemical analysis of macrophage infiltration and foam cell accumulation by CD68 staining (table 1) showed that there was a significant correlation between the extent of macrophage infiltration and foam cell accumulation (p < 0.05). There was, however, no significant association between macrophage infiltration and presence of intralesional C pneumoniae or MMP-9. There was also no significant association between foam cell accumulation and C pneumoniae infection.
Identification of C pneumoniae genomic DNA and MMP-9 transcripts in human coronary artery specimens
Nested PCR analysis identified the presence of C pneumoniae genomic DNA in four of 31 specimens (13%). Although this detection rate is lower than that of the immunohistochemical analysis, all four samples that were positive by nested PCR were also positive by C pneumoniae immunostaining. RT-PCR analysis of total cellular RNA showed the presence of MMP-9 transcripts in 16 of 28 specimens (57%). Comparison of the RT-PCR and immunohistochemical detection of MMP-9 showed a significant linear trend (p = 0.024). All four specimens positive for C pneumoniae by nested PCR showed MMP-9 production by both RT-PCR and immunohistochemistry.
DISCUSSION
This study provides evidence of an association between increased MMP-9 production and the presence of C pneumoniae in human coronary atherosclerosis and may provide evidence for a potential mechanism by which the bacteria may accelerate or exacerbate coronary atherosclerotic disease.
C pneumoniae has been associated with atherosclerosis and coronary artery disease, although the mechanism by which the bacterium may affect disease initiation or progression is poorly understood. Recent evidence shows that C pneumoniae can infect, survive in, and stimulate macrophages, smooth muscle cells, and endothelial cells,27,28 resulting in foam cell formation and MMP-9 production by macrophages in vitro.15,21,22,29,30 MMP-9 is an important contributing factor to atherosclerotic disease and is upregulated in atherosclerotic plaques, particularly during remodelling, in unstable plaques and in macrophage, foam cell, and smooth muscle cell rich areas. It is suggested that MMP-9 is associated with exacerbation of atherosclerosis and advancement of disease by breaking down matrix components and by inducing instability and rupture of the fibrous cap.
The data described here suggest that there is a strong association between the presence of C pneumoniae and MMP-9 production. A significant association was found between the extent of intralesional C pneumoniae and the amount of MMP-9 production. When serial sections of tissue were examined, C pneumoniae and MMP-9 were co-localised to the same regions of the plaque. There was no evidence in these specimens to suggest that C pneumoniae is associated with increased macrophage infiltration or foam cell formation. As MMP-9 is expressed by macrophages as well as smooth muscle cells it was not possible to ascribe the increase in MMP-9 expression to macrophage infiltration alone. The use of immunohistochemical as well as molecular techniques allowed confirmation of the association between infection with C pneumoniae and upregulation of MMP-9 transcription. Although a proportionally lower number of samples was found positive by C pneumoniae PCR, this is a finding well documented by others2 and may reflect rapid degradation of chlamydial genomic DNA and persistence of chlamydial membrane proteins within the plaque, a phenomenon that has been shown to occur with the use of in situ DNA nick end labelling (terminal deoxynucleotidyl transferase-mediated dUDP nick end labelling).31
The results of the work described here are in agreement with previously published findings. C pneumoniae has been shown to increase the capacity of macrophages to produce MMP-9 by stimulating the expression of this enzyme in human monocyte derived macrophages.22 In mouse macrophages, C pneumoniae and its 60 kDa heat shock protein stimulate tumour necrosis factor α and MMP-9 production.21C pneumoniae has been shown to activate the nuclear factor κB pathway in vascular smooth muscle cells and endothelial cells.32 In a study by Song and colleagues,33 in four atherosclerotic aortic tissue specimens immunoreactivity for MMP-9 and cyclooxygenase-2 co-localised with immunoreactivity for C pneumoniae. Choi and colleagues34 recently observed expression of MMP-9 co-localised with C pneumoniae staining in diseased aorta and carotid artery specimens.
In contrast however, Petersen and colleagues35 did not observe any association between a positive DNA test for C pneumoniae and activity of MMP-2 and MMP-9 in aortic aneurysms.35 However, the authors used separate blocks of tissue for detection of C pneumoniae DNA and MMP activity. The advantage of the present study is that the MMP-9 and C pneumoniae could be detected in sequential 5 μm sections by immunohistochemistry.
In conclusion, the data presented here provide evidence of an association between increased MMP-9 production and intralesional presence of C pneumoniae in coronary atherosclerosis and may provide increased evidence for a potential mechanism by which the bacterium may accelerate or exacerbate atherosclerotic disease.
Acknowledgments
This work was funded by British Heart Foundation grant number CH/92013.
Abbreviations
MMP, matrix metalloproteinase
PCR, polymerase chain reaction
RT-PCR, reverse transcription polymerase chain reaction
REFERENCES
- 1.Ross R . Atherosclerosis: an inflammatory disease. N Engl J Med 1999;340:115–26. [DOI] [PubMed] [Google Scholar]
- 2.Wong YK, Gallagher PJ, Ward ME. Chlamydia pneumoniae and atherosclerosis. Heart 1999;81:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell LA, Kuo C. Mouse models of Chlamydia pneumoniae infection and atherosclerosis. Am Heart J 1999;138:S516–8. [DOI] [PubMed] [Google Scholar]
- 4.Muhlestein JB. Chlamydia pneumoniae-induced atherosclerosis in a rabbit model. J Infect Dis 2000;181 (suppl 3) :S505–7. [DOI] [PubMed] [Google Scholar]
- 5.Blessing E, Campbell LA, Rosenfeld ME, et al. Chlamydia pneumoniae infection accelerates hyperlipidemia induced atherosclerotic lesion development in C57BL/6J mice. Atherosclerosis 2001;158:13–7. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Leatham EW, Carrington D, et al. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation 1997;96:404–7. [DOI] [PubMed] [Google Scholar]
- 7.Gurfinkel E, Bozovich G, Beck E, et al. Treatment with the antibiotic roxithromycin in patients with acute non-Q-wave coronary syndromes: the final report of the ROXIS study. Eur Heart J 1999;20:121–7. [DOI] [PubMed] [Google Scholar]
- 8.Torgano G, Cosentini R, Mandelli C, et al. Treatment of Helicobacter pylori and Chlamydia pneumoniae infections decreases fibrinogen plasma level in patients with ischemic heart disease. Circulation 1999;99:1555–9. [DOI] [PubMed] [Google Scholar]
- 9.Stone AF, Mendall MA, Kaski JC, et al. Effect of treatment for Chlamydia pneumoniae and Helicobacter pylori on markers of inflammation and cardiac events in patients with acute coronary syndromes: South Thames trial of antibiotics in myocardial infarction and unstable angina (STAMINA). Circulation 2002;106:1219–23. [DOI] [PubMed] [Google Scholar]
- 10.Wiesli P, Czerwenka W, Meniconi A, et al. Roxithromycin treatment prevents progression of peripheral arterial occlusive disease in Chlamydia pneumoniae seropositive men: a randomized, double-blind, placebo-controlled trial. Circulation 2002;105:2646–52. [DOI] [PubMed] [Google Scholar]
- 11.Sander D, Winbeck K, Klingelhofer J, et al. Reduced progression of early carotid atherosclerosis after antibiotic treatment and Chlamydia pneumoniae seropositivity. Circulation 2002;106:2428–33. [DOI] [PubMed] [Google Scholar]
- 12.Aalto-Setala K, Laitinen K, Erkkila L, et al. Chlamydia pneumoniae does not increase atherosclerosis in the aortic root of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2001;21:578–84. [DOI] [PubMed] [Google Scholar]
- 13.Caligiuri G, Rottenberg M, Nicoletti A, et al. Chlamydia pneumoniae infection does not induce or modify atherosclerosis in mice. Circulation 2001;103:2834–8. [DOI] [PubMed] [Google Scholar]
- 14.Kuo CC, Gown AM, Benditt EP, et al. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb 1993;13:1501–4. [DOI] [PubMed] [Google Scholar]
- 15.Kalayoglu MV, Byrne GI. A Chlamydia pneumoniae component that induces macrophage foam cell formation is chlamydial lipopolysaccharide. Infect Immun 1998;66:5067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis 1998;177:725–9. [DOI] [PubMed] [Google Scholar]
- 17.Brown DL, Hibbs MS, Kearney M, et al. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions: association of active enzyme synthesis with unstable angina. Circulation 1995;91:2125–31. [DOI] [PubMed] [Google Scholar]
- 18.Pasterkamp G, Schoneveld AH, Hijnen DJ, et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis 2000;150:245–53. [DOI] [PubMed] [Google Scholar]
- 19.Galis ZS, Sukhova GK, Lark MW, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godin D, Ivan E, Johnson C, et al. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation 2000;102:2861–6. [DOI] [PubMed] [Google Scholar]
- 21.Kol A, Sukhova GK, Lichtman AH, et al. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 1998;98:300–7. [DOI] [PubMed] [Google Scholar]
- 22.Vehmaan-Kreula P, Puolakkainen M, Sarvas M, et al. Chlamydia pneumoniae proteins induce secretion of the 92-kDa gelatinase by human monocyte- derived macrophages. Arterioscler Thromb Vasc Biol 2001;21:E1–8. [DOI] [PubMed] [Google Scholar]
- 23.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis: a report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–74. [DOI] [PubMed] [Google Scholar]
- 24.Tong CY, Sillis M. Detection of Chlamydia pneumoniae and Chlamydia psittaci in sputum samples by PCR. J Clin Pathol 1993;46:313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahon NG, Zal B, Arno G, et al. Absence of viral nucleic acids in early and late dilated cardiomyopathy. Heart 2001;86:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giambernardi TA, Grant GM, Taylor GP, et al. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol 1998;16:483–96. [DOI] [PubMed] [Google Scholar]
- 27.Godzik KL, O’Brien ER, Wang SK, et al. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol 1995;33:2411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaydos CA, Summersgill JT, Sahney NN, et al. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun 1996;64:1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalayoglu MV, Byrne GI. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis 1998;177:725–9. [DOI] [PubMed] [Google Scholar]
- 30.Kol A, Bourcier T, Lichtman AH, et al. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest 1999;103:571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer A, Roholl PJ, Gielis-Proper SK, et al. Chlamydia pneumoniae antigens, rather than viable bacteria, persist in atherosclerotic lesions. J Clin Pathol 2000;53:911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dechend R, Maass M, Gieffers J, et al. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-kappaB and induces tissue factor and PAI-1 expression: a potential link to accelerated arteriosclerosis. Circulation 1999;100:1369–73. [DOI] [PubMed] [Google Scholar]
- 33.Song YG, Kwon HM, Kim JM, et al. Serologic and histopathologic study of Chlamydia pneumoniae infection in atherosclerosis: a possible pathogenetic mechanism of atherosclerosis induced Chlamydia pneumoniae. Yonsei Med J 2000;41:319–27. [DOI] [PubMed] [Google Scholar]
- 34.Choi EY, Kim D, Hong BK, et al. Upregulation of extracellular matrix metalloproteinase inducer (EMMPRIN) and gelatinases in human atherosclerosis infected with Chlamydia pneumoniae: the potential role of Chlamydia pneumoniae infection in the progression of atherosclerosis. Exp Mol Med 2002;34:391–400. [DOI] [PubMed] [Google Scholar]
- 35.Petersen E, Boman J, Wagberg F, et al. Presence of Chlamydia pneumoniae in abdominal aortic aneurysms is not associated with increased activity of matrix metalloproteinases. Eur J Vasc Endovasc Surg 2002;24:365–9. [DOI] [PubMed] [Google Scholar]