Figure 4.
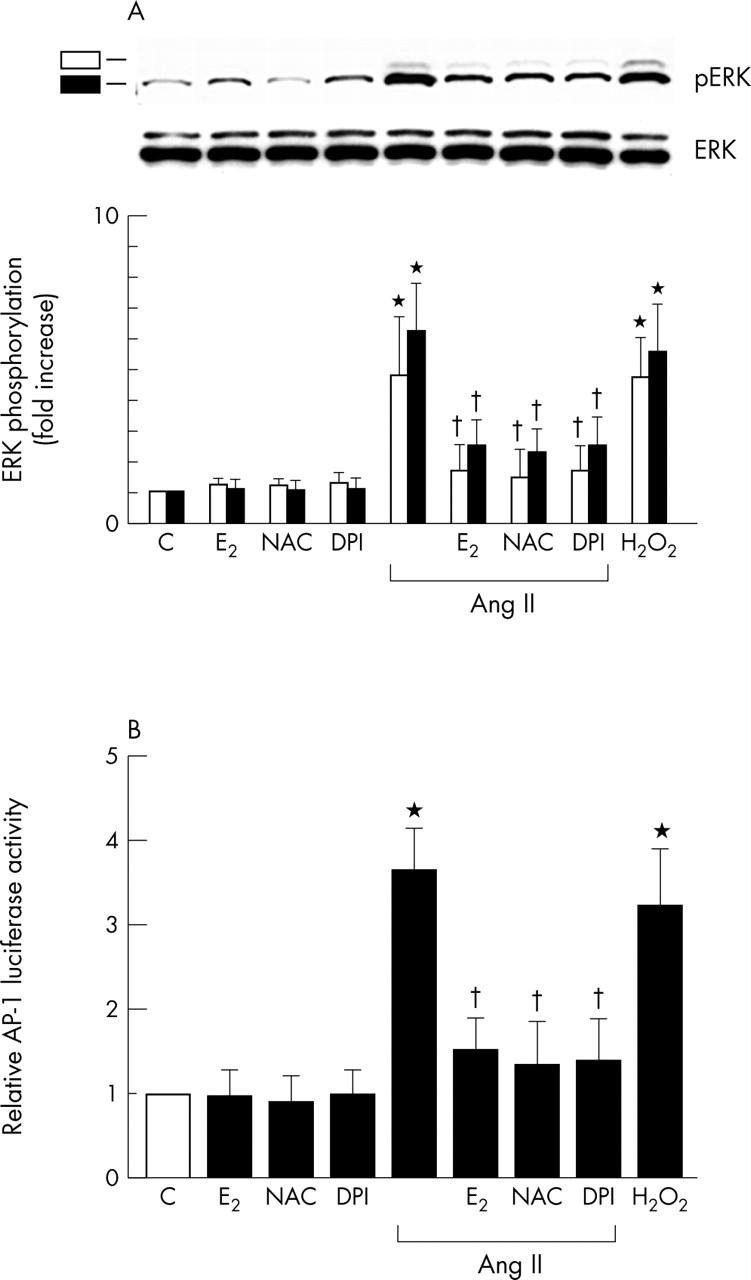
Inhibitory effect of E2 on Ang II increased extracellular signal regulated kinase (ERK) phosphorylation and activator protein 1 (AP-1) mediated reporter activity in cardiac fibroblasts. Results are shown as the mean (SEM) (n = 6). *p < 0.05 v control; †p < 0.05 v Ang II alone. (pERK, phosphorylated ERK.) (A) Effects of E2, E2 plus ICI, or various antioxidants on Ang II increased ERK phosphorylation. Cells were preincubated with E2 (100 nmol/l), NAC (10 mmol/l), or DPI (10 μmol/l) and then stimulated with Ang II (100 nmol/l) for 30 minutes. E2, NAC, or DPI inhibited Ang II induced phosphorylation of ERK. H2O2 (100 μmol/l) was used as a positive control. Phosphorylation of ERK was detected by western blotting with phospho-ERK antibody. Density was measured with a densitometer. Data are shown as fold increase relative to control groups. (B) Effects of E2, E2 plus ICI, or various antioxidants on Ang II increased AP-1 mediated reporter activity. Cardiac fibroblasts, transfected with AP-1-luciferase, were treated as indicated. Cells were preincubated with E2 (100 nmol/l), the combination of E2 plus ICI (1 µmol/l), or the antioxidant NAC (10 mmol/l) or DPI (10 μmol/l) and then stimulated with Ang II (100 nmol/l) for 24 hours or not. H2O2 (100 μmol/l) was used as a positive control. Luciferase activity was expressed as activity relative to untreated C.
