Abstract
The ability of monkeys to follow the gaze of other individuals is a matter of debate in many behavioral studies. Physiological studies have shown that in monkeys, as in humans, there are neural correlates of eye direction detection. There is little evidence at the behavioral level, however, of the presence and development of such abilities in monkeys. The aim of the present study was to assess in juveniles and adult pig-tailed macaques (Macaca nemestrina) the capacity to use eye cues only to follow the gaze of an experimenter. Biological stimuli (head, eye, and trunk movements) were presented by an experimenter to 2 adult monkeys with their heads restrained (Experiment 1) and to 11 monkeys of different ages, free to move in their home cages (Experiment 2). A nonbiological stimulus served as a control. Results showed that macaques can follow the gaze of the experimenter by using head/eye and eye cues alone. Trunk movements and nonbiological stimuli did not significantly elicit similar reactions. Juvenile monkeys were not able to orient their attention on the basis of eye cues alone. In general, gaze following was more frequent in adults than in juveniles. Like in humans, however, such abilities in macaques dramatically improve with age suggesting that the transition to adulthood is a crucial period in the development of gaze-following behavior.
Gaze following is defined as the ability of one individual (X) to follow the direction of gaze of a second individual (Y) to a location in space (1). The ability to track visually the gaze direction of conspecifics to targets may have a considerable adaptive advantage, because individuals can gain information about food sources, social status of conspecifics, and the locations of predators (2, 3). It has been proposed that gaze perception plays a crucial role in social interactions (3–5).
It is well established that apes (chimpanzees and orangutans) are able to follow the gaze direction of conspecifics (5) and humans (6, 7) by using a combination of head-and-eyes stimuli. Chimpanzees are also able to follow the gaze direction of a human experimenter by observing the eye direction alone, independently of the head movement (6–8). In addition, chimpanzees and one orangutan have been reported to use eye cues from the experimenter to perform successfully object-choice tasks (9). To date, there is no evidence that primates, other than chimpanzees, orangutans, and humans, can use eye gaze alone to monitor where a human observer is looking. In monkeys, the ability to follow the eye gaze alone has not been substantiated empirically. Recent observations have shown, however, that monkeys can orient their attention in response to static pictures of conspecifics looking toward specific directions (10).
Neurophysiological studies have provided evidence that neural mechanisms coding the direction of the eyes exist in monkeys. Single-unit studies have shown that in the superior temporal sulcus of the macaque monkey there are neurons sensitive to the orientation of face and eyes (11, 12). Perrett et al. (12) have suggested that one function of such cells would be to assist in the recognition of where another individual is looking. Functional MRI studies in humans demonstrated an activation of the superior temporal sulcus region during the observation of eye-gaze direction of other individuals (13, 14). This region is probably homologous to the superior temporal sulcus region of monkeys studied by Perrett et al. (12). Altogether these findings demonstrated that humans and monkeys possess brain mechanisms coding eye gaze and suggested that gaze-following behavior based on eye cues only could be present not only in humans but also in monkeys.
In recent years, the analysis of gaze direction has been considered from a cognitive and developmental perspective. According to some authors, the observation and interpretation of gaze may be a key factor in developing a “theory of mind” (ToM; refs. 15–17)—the capacity to attribute mental states, such as intentions or beliefs, to other individuals (15, 18). In humans, gaze-following behavior emerges between 3 and 18 months (19–22) of age. By 14–18 months of age, infants follow the gaze of adults by using eye cues only (23), and by 18-to-24 months, they are able to understand the referential aspect of visual attention of others. At approximately the same age (18–24 months), infants develop other behavioral and cognitive abilities such as self-conscious emotions, planning, and the capacity to recognize themselves in mirrors (6). It is not until the age of 4 years, however, that they are able to perform successfully ToM tasks by using others' gaze directions (24–26). Taken together, all these investigations indicate that, during human ontogenetic development, gaze-following behavior precedes the establishment of a ToM. Although it is not clear whether gaze following is linked to the ToM by a cause-effect relationship, it is possible that it constitutes a precursor or a marker during the establishment of a mind-reading system. Except for the study by Povinelli and Eddy (27), which indicates young chimpanzees are able to follow the gaze of other individuals by the age of at least 5 years, no empirical works have considered developmental factors linked to the ability of gaze following in nonhuman primates.
In this article, we report two experiments in which we tested the hypothesis that the observation of the direction of eye gaze will elicit a gaze-following response (GFR) in macaques. In the first experiment, we tested this hypothesis by assessing the gaze response of two head-restrained macaques while head/eye or eye stimuli were presented by an experimenter. Gaze responses were then compared with a nonbiological stimulus that served as a control. In the second experiment, we tested the same hypothesis in a seminaturalistic setting where the monkeys were free to move in their home cages. The aim of the second experiment was also to assess, by comparing macaques of different ages, whether the gaze-following capability is age dependent, as it is in humans.
Methods
Experiment 1.
Subjects were two pig-tailed macaques, one male (M1: age 8 years) and one female (M2: age 9 years), living at the Primate Section of the Institute of Human Physiology. Both animals were born in captivity. They were housed individually in cages (100 × 160 × 100 cm) that allowed visual, auditory, and restricted tactile contact with other monkeys. The cage environment was enriched with toys and foraging boards. Because the monkeys had been already used for neurophysiological studies, they were already familiar with the testing room, primate chair, and head restriction. Both monkeys were used to the presence of and the interaction with the experimenters. During testing, the monkeys were not food deprived. In addition to primate pellets, their diet was enriched daily with fruits, vegetables, seeds, and living coleopteran larva.
Procedures.
During the test session, the monkey was seated in a primate chair with its head restrained. The experimenter sat facing the monkey at a 1.5-m distance. To determine which stimulus features elicited a GFR, the monkeys were tested in three experimental conditions: head/eyes, eye, and control. In the head/eyes condition, the experimenter turned the head 70° up, down, left, or right, with the eyes always aligned with the head (Fig. 1b). In the eye condition, the experimenter oriented the eyes up, down, left, or right to the extreme position of the orbit (Fig. 1c). In the control condition, a white painted box (26 × 20 × 15 cm) marked with two circular red spots on the front side (Fig. 1a) was placed in front of the monkey at a distance of 1.5 m. The control box was moved around horizontal and vertical axes by means of strings maneuvered from a hidden place to the four directions: up, down, left, or right. For each stimulus direction, a total of 12 trials was administered. Each trial started when the stimulus began to move. In each trial the end position was kept for 3 s. The trial was considered concluded at the end of the 3 s. Conditions and trials were administered randomly. Each monkey received one 20-min-long session. The experiment was videotaped by a Panasonic VHS video camera that was positioned behind the experimenter. A mirror placed to one side of the monkey's head and facing the experimenter allowed recording of the experimenter's gaze.
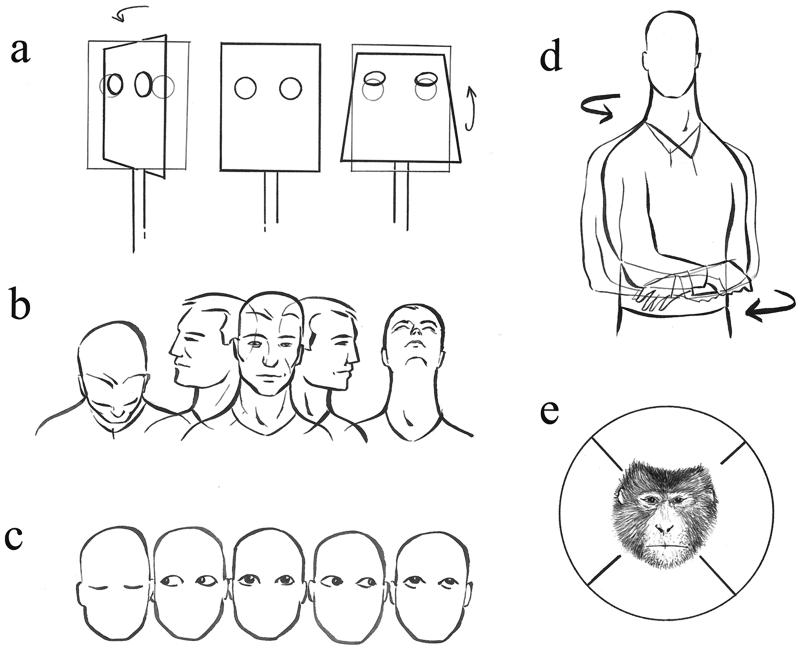
Figure 1.
Schematic illustrations of the experimental conditions. (a) Control condition. The box was moved around horizontal and vertical axes. (b) Head/eyes condition. The experimenter turned the head together with eyes about 70° up, down, left, or right. (c) Eye condition. The experimenter oriented the eyes up, down, left, or right. (d) Trunk condition. The experimenter turned the trunk to the left or to the right. (e) Possible subject's response to the stimulus. Subject's response may fall into one of these four sectors. Each sector covers an area delimited by a 90° angle, 45° clockwise and counterclockwise with respect to the stimulus direction.
Experiment 2.
Subjects were 11 pig-tailed macaques (Macaca nemestrina), 5 females and 6 males. All macaques were captive-born. Infants lived with the mother until the age of 2–3 years. Their ages ranged between 2 and 16 years. The monkeys were subdivided into two groups: juvenile/adolescent monkeys and adult monkeys. The monkeys of the first group (juveniles) had an age between 2 and 6 years. The monkeys of the second group (adults) had an age of >6 years. This distinction was made considering a series of morphological, developmental, and behavioral factors typical of macaque species (28–30). All subjects lived at the Primate Section of the Institute of Human Physiology. They were housed in single cages (cage measurements: 100 × 160 × 100 cm) of a connected-cage system that allowed brief, daily periods of interaction between two or more individuals. The environment and the diet were the same as those described for Experiment 1.
All experiments complied with the European law on the humane care and use of laboratory animals.
Procedures.
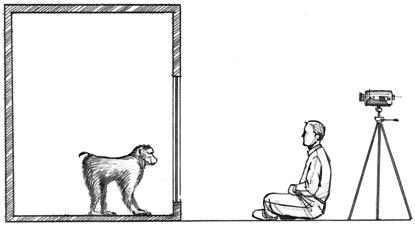
During the test session, the monkey was separated from other individuals. The experimenter sat at a 1.5-m distance in front of the cage (see Fig. 2). A curtain positioned behind the experimenter prevented the monkey from interacting with other individuals or objects while tested. To determine which stimulus features could elicit a GFR, we administered four experimental conditions. The first three conditions were the same as those described in Experiment 1. The fourth was a trunk condition that required the experimenter to turn his trunk about 60° to the left or to the right (Fig. 1d). In each trial, the turned position lasted 3 s. Condition and trials were administered randomly. For each stimulus direction, a total of 12 trials was administered. Each subject was tested during two sessions performed on different days. The experiment was videotaped by a video camera positioned behind the experimenter (Fig. 2). A mirror placed on the front of the cage faced the experimenter and allowed for the recording of the experimenter's gaze.
Figure 2.
Schematic illustration of the experimental setting. The experimenter sat facing the monkey at a 1.5-m distance. A video camera placed behind the experimenter videotaped each session. A mirror placed on the front of the cage faced the experimenter and allowed the recording of the experimenter's gaze.
Behavioral Analysis of Experiments 1 and 2.
Videotapes were analyzed independently by two experimenters. To judge the monkeys' responses to the movements of the experimenter, the directions of monkeys' gazes were subdivided into four classes: up, down, left, or right (Fig. 1e). A trial was considered valid if the monkey engaged its gaze with the stimulus at the trial beginning. If this criterion was met, the direction of the first gaze movement after stimulus visual engagement was recorded. In Experiment 1, the gaze movement made by the monkey referred to the movement of the eyes only, because the head was restrained. If the monkey did not look at the stimulus during the trial, then the subject's response was considered invalid and not included in the statistical analysis. GFR was scored if the subject directed its gaze toward the sector corresponding to the stimulus direction. No GFR was scored if the subject directed its gaze toward a sector different from that of the stimulus direction or if the monkey did not move its gaze.
To control for possible effects caused by differences in attention between juveniles and adults, 5 min of behavioral analysis was scored randomly during each test session. The behaviors analyzed were (i) amount of time in which the monkey's body was oriented toward the experimenter and (ii) amount of time in which the monkey was visually exploring the experimenter.
A third scorer was included to determine the interobserver reliability that was calculated for a sample of 117 trials. The average reliability of the three resulting pairwise comparisons was 92.4% (range = 91.3%–94.8%).
To check whether the vision of the experimenter showing the stimulus could bias the scorer, two naive scorers analyzed a sample of 103 trials in which the vision of the experimenter on the screen was prevented. We then compared the number of discrepancies between blind scoring and what the actual scorers judged when scoring the 103 trials. The percentage of agreements of the two different methodological scoring samples was close to 100% (scorer 1, 96.01%; scorer 2, 94.11%), thus demonstrating the high reliability of judgements made during the nonblind scoring.
Statistical Analysis.
For Experiment 1, the number of GFRs divided by the total number of valid trials was recorded and compared with that expected by chance (expected probability of 0.25) by using the binomial test. Data were analyzed further by plotting together results from the two subjects and comparing eye and head/eyes conditions to control by means of the McNemar nonparametric test.
For Experiment 2, each monkey's percentage of GFRs (number of GFRs divided by the total number of valid trials in each experimental condition) was calculated for each condition in the two sessions. Because statistical analysis (Wilcoxon matched-pairs test) did not reveal any significant difference between the two sessions for each condition, we averaged the results of the two sessions. The experimental condition was then compared with the control condition by using the Wilcoxon matched-pairs test. The Mann–Whitney test was applied for adult versus juvenile comparisons.
Spearman's rank order correlation analysis was carried out to correlate the ages of the animals and the percentages of GFRs. Behavior of adults and juveniles (i.e., time with the body oriented toward the experimenter and time inspecting the experimenter) was compared by a two-way ANOVA (2 × 4 ANOVA with groups of different ages and sectors to where gaze was directed, as main factors). If ANOVA did reveal significant differences between the two groups, post hoc testing was performed by using probable least-squares difference tests.
To determine at which age GFR is present, each monkey's number of GFRs was compared with that expected by chance (expected probability of 0.25) by using the binomial test. The expected probability of 0.25 is also confirmed by the percentage of responses during invalid trials displayed by animals at the stimulus presentation. Behavioral analysis (5 min) was selected randomly during each test session, and all responses in invalid trials were recorded. As a result of this behavioral analysis, data showed that during invalid trials, the directional responses of animals were equally distributed in the four spatial directions (percentage means are given): up, 24.03; down, 26.17; left, 26.61; right, 23.01; nonsignificant, McNemar test.
Results
Experiment 1.
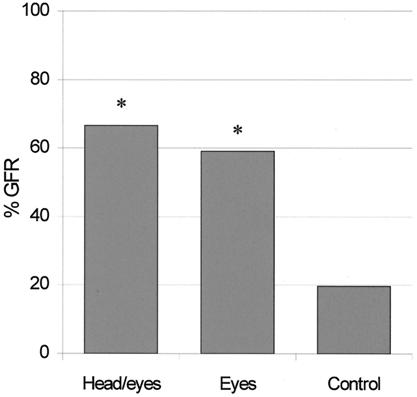
Fig. 3 illustrates the results of the experiment. Both subjects made a number of GFRs greater than chance either when the stimulus was the head/eyes (M1, 17/22, P < 0.00001; M2, 15/26, P < 0.0005) or the eyes alone (M1, 12/15, P < 0.00005; M2, 14/29, P < 0.005). No differences were observed in the control condition (M1, 6/39; M2, 7/27). Statistical analysis showed that the observation of the head/eyes and of the eyes alone elicits a higher proportion of GFR than the control (P < 0.005 and P < 0.05, respectively).
Figure 3.
Percentage of GFR as a function of the experimental condition. Data represent the mean between the two monkeys. Asterisks indicate the significance of each condition compared with control (*, P < 0.05).
The valid trials were equally distributed among the four spatial directions. After calculating each spatial direction in the three conditions, the percentages of valid trials ranged from 19.4% to 34% (nonsignificant, McNemar test).
Experiment 2.
Fig. 4 illustrates the results of the experiment. For adults, statistical analysis showed a significant difference in head/eyes and eye-gaze following compared with control (z = 2.20, P < 0.05). For juveniles, statistical analysis showed a significant difference in percentage of GFRs elicited by the head/eyes cues compared with control (z = 2.02, P < 0.05). Eye cues only were not able to elicit a GFR in juveniles. When compared with adults, juveniles showed a lower percentage of GFR when the stimuli were both the head/eyes or the eyes (P < 0.01). Binomial tests revealed that the number of GFRs in the head/eyes condition is significantly above chance at the age of 3 (P < 0.05), 4 (P < 0.05), 5 (P < 0.00001), and 6 (P < 0.005) and in all adults (levels of significance ranging from P < 0.005 to P < 1 × 10−16), but it is not significant in the 2-year-old monkey. Binomial tests also revealed that the number of GFRs in the eyes condition is significantly above chance at the age of 5 (P < 0.05) and in all adults (levels of significance ranging from P < 0.01 to P < 1 × 10−7).
Figure 4.
Percentage of trials in which juvenile and adult monkeys showed GFR as a function of experimental condition. The data recorded of each monkey during the two sessions were averaged and plotted together. Each bar represents the average of the percentage of GFRs in adults and juveniles. * indicates the significance of the comparison between experimental condition and control (P < 0.05). ** indicates the significance of the comparison between experimental condition and control (P < 0.05) and between adults and juveniles (P < 0.01).
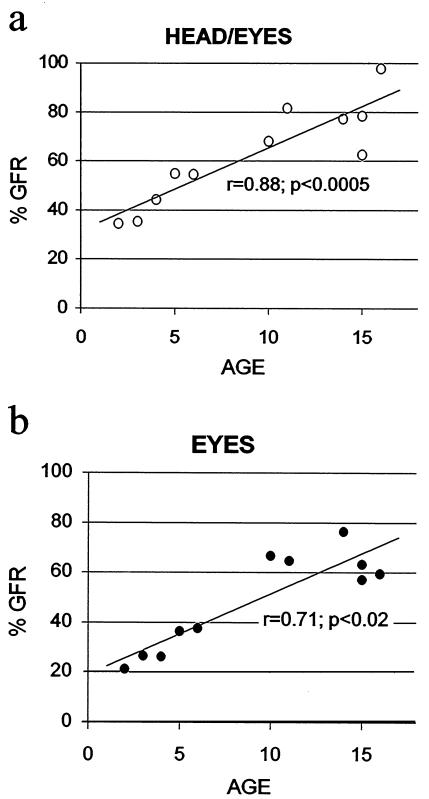
The percentages of head/eyes- (Fig. 5a) and eyes alone- (Fig. 5b) gaze following as a function of age are illustrated in Fig. 5. Statistical analysis revealed a significant correlation between age and the percent of trials eliciting GFR in response to head/eyes (r = 0.88, P < 0.0005) and eyes alone movements (r = 0.71, P < 0.05).
Figure 5.
Percentages of head/eyes (a) and eyes alone (b) GFRs as a function of age.
The statistical comparison between adults and juveniles showed the difference in GFR was not caused by attentional differences between the two groups. In fact, ANOVA did not show any statistical differences between the two groups. During 5 min of behavioral analysis scored randomly during the test session, adults and juveniles spent a similar amount of time with the body oriented toward the experimenter (data expressed as means ± SEM; adults, 257.7 ± 9.9 s; juveniles, 240.2 ± 10.5 s) and a similar amount of time inspecting the experimenter (adults, 16.2 ± 21.7 s; juveniles, 19.2 ± 15.7 s).
Discussion
The issue of whether nonhuman primates are able to follow the gaze of conspecifics has been the aim of many ethological investigations. There is general accord that apes possess this ability, but whether this ability also applies to monkeys is still a matter of debate. There is evidence that macaques follow the gaze by using a combination of head-and-eyes stimuli spontaneously provided by a conspecific in a naturalistic situation (5), by observing a videotape (1), or by observing a human experimenter (31). In contrast, Itakura (7), in a study in which he compared 11 species of nonhuman primates, reported that apes show GFR above chance, whereas monkeys do not. Up to now, only one study explored the possibility that gaze following in monkeys can be elicited by eye cues alone (10), although stimuli were presented to head-restrained monkeys by means of static pictures.
The results of the two experiments presented here demonstrate that macaques are able to use head/eye cues to follow the gaze either in a head-restrained condition or in a free-moving condition. In contrast, nonbiological stimuli (control) and movements of the trunk oriented to different locations in space do not elicit a significant GFR. Our data also demonstrate that eye cues alone may play a key role in eliciting GFRs, thus extending to monkeys what was considered to be unique to humans and apes.
Other investigations showed that the facial region, specifically the eye region, is the preferential target of monkeys observing conspecific facial images (32, 33). The periocular region is used in social contexts to convey information about the emotional state of the individual, and thus it can be used to communicate affiliative or agonistic intentions or to recognize them when produced by other individuals (32, 34). Because social communication in nonhuman primates heavily depends on facial expressions, it is conceivable that gaze-following behavior based on eye cues has evolved by “exploiting” the natural tendency of monkeys to observe salient facial features such as the eyes.
The results of the second experiment showed that, as in humans, GFR in macaques dramatically improves with age. Compared with adults, juvenile monkeys showed a marked difference in head-gaze following, because they were unable to determine the direction of another's gaze by using eye cues only. These results cannot be explained on the basis of differences in attentional factors, because adults and juveniles devoted the same amount of time interacting with and visually exploring the experimenter. In juveniles, the orientation of the head and eyes together is the first feature that triggers a shift in visual attention response suggesting that in young macaques head-and-eyes orientation together provide more salient signals to the direction of another's gaze than eyes alone. This ontogenetic trend resembles that of humans: 3- to 6-month-old infants are able to follow the gaze of an adult by using a combination of head-and-eye cues, but it is not until 14–18 months that they are able to follow the gaze by using eye cues only (19, 21, 23). Thus, in humans, this ability develops when children are still dependent on their parents. In monkeys, GFR based on head/eye cues develops between the 2nd and the 4th years, a stage at which juveniles are weaned but still socially dependent on their mothers (30, 35). According to our results, however, GFR based on eye cues alone seems to develop only at a later stage. This finding renders unlikely the interpretation that GFR is related to the maturation of visuomotor coordination and motor skills, because in monkeys, these skills are settled well before the end of the juvenile and adolescent period. It is therefore possible to hypothesize that the development of GFR could be related to the relatively long period preceding adulthood, during which young macaques have the opportunity to explore better the physical environment (35, 36) and to learn the rules of their complex social context (28). Indeed, it is well known that juveniles spend much of their nonfeeding time engaging in social play. It has been proposed that through play, juveniles may establish dominance relationships and learn social and communication skills (28). Thus, it seems that in macaques, the emergence of a GFR based on head-and-eye cues could be linked to the processes of transition to adulthood when individuals acquire skills and develop relationships that may be of both immediate and long-term benefit (28, 36).
A final comment for discussion in light of the present results is the relation of GFR to the appearance of cognitive processes leading to the development of a ToM. The link between gaze following and the ToM was theorized by Baron-Cohen et al. (37). According to these authors, the perception of eye gaze is a crucial step to the development of a mind-reading system that allows individuals to understand not only what another individual is attending to, but also what it is thinking about. Thus, the eyes can be thought of as “windows” to the minds of others. From an evolutionary perspective, some authors have proposed that the ToM evolved to cope with social challenges (3). In accord with this proposal, several investigations in humans showed that deficits in gaze following are associated with impairments in social and cognitive abilities such as autism (37). Our results do not allow us to conclude that a ToM does exist in monkeys. However, the fact that macaques, together with humans and great apes, display the ability to follow the gaze of others by using eye cues corroborates the notion of “cognitive continuity” in the domain of intentional-state attribution among different primate species (see ref. 38 for a broader discussion and ref. 39).
Acknowledgments
We thank Prof. G. Rizzolatti and Dr. E. Visalberghi for their valuable comments and criticisms on an early draft of the manuscript. We also thank the two anonymous referees for their constructive criticism of our manuscript. We thank G. Braghiroli for the excellent work in drawing the illustrations of Figs. 1 and 2. This study was supported by a Human Frontier Science Program Organization grant (to L.F. and V.G.), the Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica, and the Swiss National Science Foundation.
Abbreviations
- ToM
theory of mind
- GFR
gaze-following response
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.250241197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.250241197
References
- 1.Emery N J, Lorincz E N, Perrett D I, Oram M W, Baker C I. J Comp Psychol. 1997;111:286–293. doi: 10.1037/0735-7036.111.3.286. [DOI] [PubMed] [Google Scholar]
- 2.Menzel E W, Halperin S. Science. 1975;189:652–654. doi: 10.1126/science.1162352. [DOI] [PubMed] [Google Scholar]
- 3.Whiten A, Byrne R W. In: Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes and Humans. Byrne R W, Whiten A, editors. Oxford: Oxford Univ. Press; 1988. pp. 211–223. [Google Scholar]
- 4.Thomsen C E. Anim Behav. 1974;22:144–149. [Google Scholar]
- 5.Tomasello M, Call J, Hare B. Anim Behav. 1998;55:1063–1069. doi: 10.1006/anbe.1997.0636. [DOI] [PubMed] [Google Scholar]
- 6.Povinelli D J, Eddy T J. Psychol Sci. 1996;7:129–135. [Google Scholar]
- 7.Itakura S. Jpn Psychol Res. 1996;38:174–180. [Google Scholar]
- 8.Tomasello M, Hare B, Agnetta B. Anim Behav. 1999;58:769–777. doi: 10.1006/anbe.1999.1192. [DOI] [PubMed] [Google Scholar]
- 9.Itakura S, Tanaka M. J Comp Psychol. 1998;112:119–126. doi: 10.1037/0735-7036.112.2.119. [DOI] [PubMed] [Google Scholar]
- 10.Lorincz E N, Baker C I, Perrett D I. Curr Psychol Cogn. 1999;18:973–1003. [Google Scholar]
- 11.Perrett D I, Smith P A J, Potter D D, Mistlin A J, Head A S, Milner A D, Jeeves M A. Proc R Soc London. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- 12.Perrett D I, Hietanen J K, Oram M W, Benson P J. Philos Trans R Soc London. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- 13.Puce A, Allison T, Bentin S, Gore J C, McCarthy G M. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman E A, Haxby J V. Nat Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- 15.Baron-Cohen S. Curr Psychol Cogn. 1994;13:513–552. [Google Scholar]
- 16.Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: Massachusetts Institute of Technology Press; 1995. [Google Scholar]
- 17.Langton S R H, Watt R J, Bruce V. Trends Cogn Sci. 2000;4:50–59. doi: 10.1016/s1364-6613(99)01436-9. [DOI] [PubMed] [Google Scholar]
- 18.Premack D, Woodruff G. Behav Brain Sci. 1978;1:515–526. [Google Scholar]
- 19.Scaife M, Bruner J S. Nature (London) 1975;253:265–266. doi: 10.1038/253265a0. [DOI] [PubMed] [Google Scholar]
- 20.Butterworth G, Cochran E. Int J Behav Dev. 1980;3:253–272. [Google Scholar]
- 21.Butterworth G, Jarret N. Br J Dev Psychol. 1991;9:55–72. [Google Scholar]
- 22.Corkum V, Moore C. In: Joint Attention: Its Origins and Role in Development. Moore C, Dunham P J, editors. Hillsdale, NJ: Erlbaum; 1995. pp. 61–83. [Google Scholar]
- 23.Moore C, Corkum V. Br J Psychol. 1998;16:495–503. [Google Scholar]
- 24.Baron-Cohen S. In: Joint Attention: Its Origins and Role in Development. Moore C, Dunham P J, editors. Hillsdale, NJ: Erlbaum; 1995. pp. 41–59. [Google Scholar]
- 25.Perner J. Understanding the Representational Mind. Cambridge, MA: Massachusetts Institute of Technology Press; 1992. [Google Scholar]
- 26.Wellman H M. The Child's Theory of Mind. Cambridge, MA: Massachusetts Institute of Technology Press; 1990. [Google Scholar]
- 27.Povinelli D J, Eddy T J. Curr Psychol Cogn. 1994;13:695–705. [Google Scholar]
- 28.Walters J R. In: Primate Societies. Smuts B B, Cheney D L, Seyfarth R M, Wrangham R W, Struhsaker T T, editors. Chicago: Univ. of Chicago Press; 1987. pp. 358–369. [Google Scholar]
- 29.Swindler D R. Introduction to the Primates. Seattle: Univ. of Washington Press; 1998. [Google Scholar]
- 30.Fa J E, Lindburg D G. Evolution and Ecology of Macaque Societies. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 31.Anderson J R, Mitchell R W. Folia Primatol Basel. 1999;70:17–22. doi: 10.1159/000021670. [DOI] [PubMed] [Google Scholar]
- 32.Nahm F K D, Perret A, Amaral D G, Albright T D. J Cognit Neurosci. 1997;9:611–623. doi: 10.1162/jocn.1997.9.5.611. [DOI] [PubMed] [Google Scholar]
- 33.Wilson F W W, Goldman-Rakic P S. Behav Brain Res. 1994;60:79–89. doi: 10.1016/0166-4328(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 34.Maestripieri D. Behavior. 1996;133:997–1022. [Google Scholar]
- 35.Smuts B B, Cheney D L, Seyfarth R M, Wrangham R W, Struhsaker T T. Primate Societies. Chicago: Univ. of Chicago Press; 1987. [Google Scholar]
- 36.Janson C H, van Schaik C P. In: Juvenile Primates. Life History, Development, and Behavior. Pereira M E, Fairbanks L A, editors. Oxford, U.K.: Oxford Univ. Press; 1993. pp. 57–74. [Google Scholar]
- 37.Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J. Br J Dev Psychol. 1995;13:379–398. [Google Scholar]
- 38.Tomasello M, Call J. Primate Cognition. Oxford, U.K.: Oxford Univ. Press; 1997. [Google Scholar]
- 39.Gallese V, Goldman A. Trends Cogn Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]