Abstract
A 40 year old patient presented with acute anterior wall infarction. A non-calcified lesion was stented directly without significant expansion of the stent. Rotational atherectomy successfully removed parts of the maldeployed stent and resistant arterial wall substance allowing full dilatation of the lesion.
Keywords: complications, direct stenting, non-expanded stent, rotablation
Direct coronary stent implantation, defined as stenting without prior balloon dilatation, is an elegant technique for coronary artery revascularisation with many advantages. One of the major periprocedural complications of direct stenting is the lack of full deployment of the stent,1 meaning that the residual stenosis after stent implantation is more then 30%. Complex (B2, C) calcified lesions in small vessels limit use of this technique.2 Here, we report a case of an underdeployed stent and how we managed this complication in a man with acute anterior wall myocardial infarction.
CASE REPORT
A 40 year old patient was admitted to our coronary care unit because of acute coronary syndrome with ST segment elevation in the precordial leads (V1–V4). The patient presented no sign of heart failure (Killip class I). He had suffered a myocardial infarction a few years previously, but he had not undergone coronary angiography or coronary revascularisation thereafter. He had multiple risk factors for cardiovascular disease: a long history of smoking, hypertension, hypercholesterolaemia, and a positive family history for coronary artery disease.
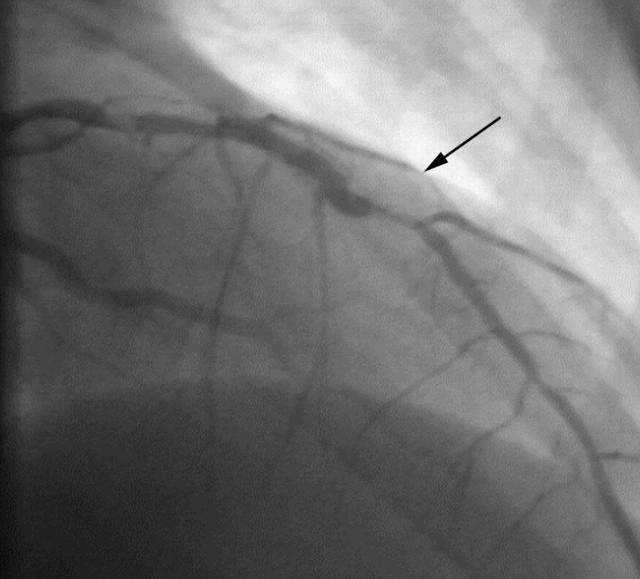
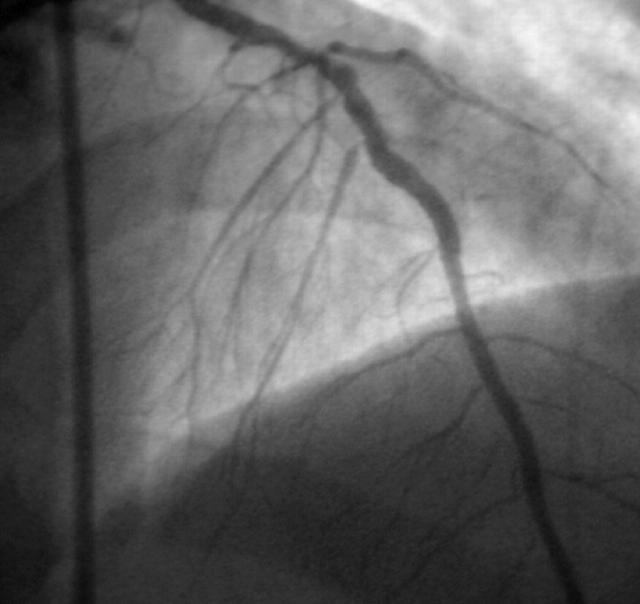
He was immediately transferred to the catheterisation laboratory. Coronary angiography showed an ulcerated plaque in the beginning of the mid segment of the left anterior descending artery (LAD), with additional lesions further down this vessel (fig 1). No calcium deposits were noticed on fluoroscopy. Therefore, direct stent implantation was planned for all of the three significant stenoses. Firstly, a cobalt-chromium stent (3.5 × 12 mm) was implanted in the most proximal lesion without any problems.
Figure 1.
Initial angiogram showing three significant stenoses (one is indicated by the arrow).
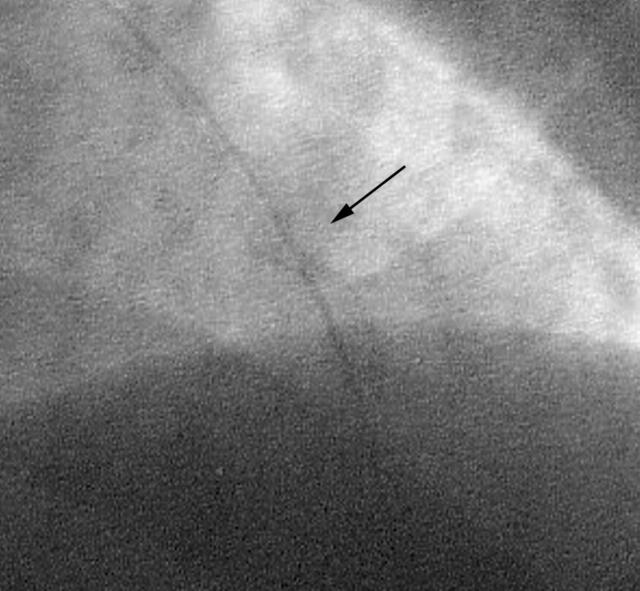
A cobalt-chromium stent (3.0 × 18 mm) was then advanced into the mid lesion. Unfortunately, the lesion and the stent resisted significant dilatation. Subsequent dilatations with various semicompliant and high pressure balloons up to 30 atm of pressure could not expand the stent further (fig 2).
Figure 2.
Underexpanded stent (arrow).
Therefore, we decided on rotablating the lesion and with it the underdeployed stent. A 1.25 mm and 1.75 mm burr (Rotablator, Boston Scientific) was very slowly advanced through the lesion (fig 3).
Figure 3.
Rotablation of the underexpanded stent (arrow).
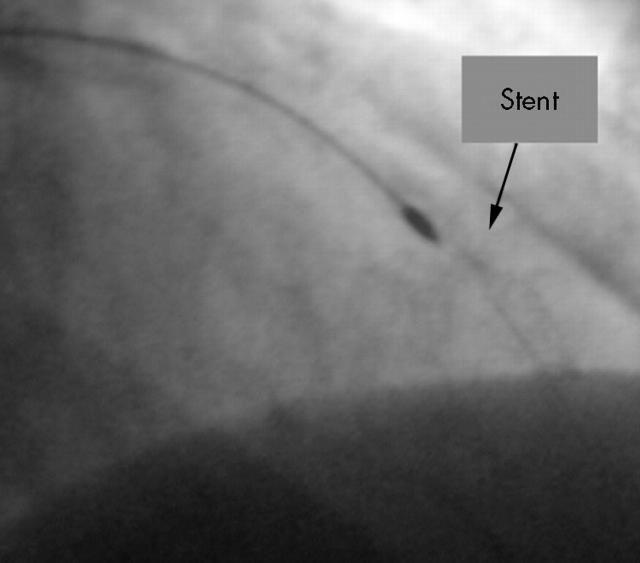
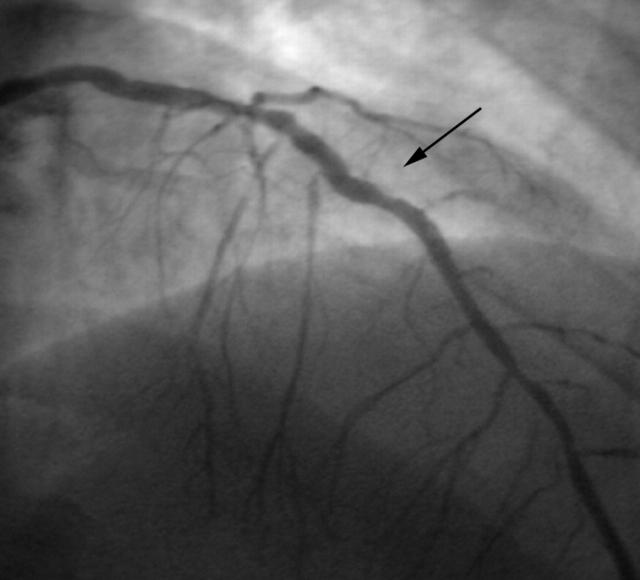
After rotablation the lesion was dilated with a conventional balloon at 14 atm of pressure. Because tissue had prolapsed into the lumen of the LAD (fig 4) a new cobalt-chromium stent (3.0 × 8 mm) was inserted into the rotablated area. Figure 5 shows the final angiogram of the patient’s LAD.
Figure 4.
After rotablation a small tissue prolapse became visible in the rotablated stent.
Figure 5.
Final angiogram after stent implantations into the distal lesion and the rotablated stent.
DISCUSSION
The development of third generation stents with a very low profile, great flexibility, and high tightness on a high pressure balloon has made direct stenting possible. Direct stenting is the implantation of stents in coronary lesions without predilatation in a high percentage of interventional cases.1–3 Initial registries have indicated a high success rate in combination with a low complication rate.1–4 From animal restenosis models, direct stenting without the need for predilatation appears to reduce vessel trauma, in particular as a result of less endothelial denudation, resulting in less neointimal hyperplasia subsequently.5 However, whether this potential benefit applies also to human atherosclerotic vessels has not been proved.
Although some studies have indicated a significant reduction of restenosis after direct stenting, most angiographic studies have shown equivalency to stenting after predilatation.3,4,6,7 With respect to reduction of material costs, lower radiation exposure, and a decreased need for radiographic contrast, direct stenting appears favourable relative to conventional stenting.4
Although direct stenting is a very elegant procedure with the advantages mentioned above, underdeployment of the stent is an inherent risk. Most randomised multicentre prospective studies have defined strict criteria for this procedure.5,8,9 Eligible cases include single lesion percutaneous coronary intervention in a suitable vessel (no major calcification, no angulation proximal to the lesion, vessel size > 2.5 mm or even 3.0 mm, and lesion length no more than 25 mm). Totally occluded vessels were excluded. Univariate analysis by Chevalier et al10 identified the following predictors of failure of direct stenting: low minimum lumen diameter before percutaneous transluminal coronary angioplasty, circumflex location, distal location, use of a GFX stent (Arterial Vascular Engineering, Santa Rosa, California, USA), a calcified vessel, and age > 70 years.
In this case no calcifications of the coronary arteries in this 40 year old patient were noticeable on fluoroscopy and we have had no contraindication to direct stenting. Nevertheless, the stent could be expanded only minimally. A largely underexpanded bare metal stent is very often prone to occlusion by thrombosis or, later, to the formation of neointima. Therefore, a very reasonable way to treat this complication would have been aortocoronary bypass surgery. However, aortocoronary bypass surgery did not seem to be a rational choice of urgent treatment for this patient because bypass surgery in the setting of an acute myocardial infarction is associated with a substantial increase in periprocedural mortality.11
To our knowledge, there are no data about how to remove poorly expanded stents. Taking into account the risk of an emergency surgical revascularisation of the vessel, rotational atherectomy seemed to be the choice with a high probability of success because the device is very flexible and does not require the advancement of a housing or other non-ablating device parts into a very narrow and resistant lesion.
Conclusions
Direct coronary stenting is a material and cost saving procedure for the treatment of coronary artery disease. Since the prognosis of largely underexpanded stents is poor and the treatment of such stent situations is costly, coronary lesions for direct stenting should be carefully selected.
REFERENCES
- 1.Briguori C, Sheiban I, De Gregorio J, et al. Direct coronary stenting without predilatation. J Am Coll Cardiol 1999;34:1910–5. [DOI] [PubMed] [Google Scholar]
- 2.Brito FS, Caixeta AM, Perin MA, et al. Comparison of direct stenting versus stenting with predilatation for the treatment of selected coronary narrowings. Am J Cardiol 2002;89:115–20. [DOI] [PubMed] [Google Scholar]
- 3.Pentousis D, Guerin Y, Funck F, et al. Direct stent implantation without predilatation using the Multilink stent. Am J Cardiol 1998;82:1437–40. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Elbal L, Ruiz-Nodar JM, Zueco J, et al. Direct coronary stenting versus stenting with balloon pre-dilation: immediate and follow-up results of a multicentre, prospective, randomized study: the DISCO trial. Direct stenting of coronary arteries. Eur Heart J 2002;23:633–40. [DOI] [PubMed] [Google Scholar]
- 5.Brueck M, Scheinert D, Wortmann A, et al. Direct coronary stenting versus predilatation followed by stent placement. Am J Cardiol 2002;90:1187–92. [DOI] [PubMed] [Google Scholar]
- 6.Rogers C, Parikh S, Seifert P, et al. Endogenous cell seeding: remnant endothelium after stenting enhances vascular repair. Circulation 1996;94:2909–14. [DOI] [PubMed] [Google Scholar]
- 7.Baim DS, Flatley M, Caputo R, et al. Comparison of PRE-dilatation vs direct stenting in coronary treatment using the Medtronic AVE S670 Coronary Stent System (the PREDICT trial). Am J Cardiol 2001;88:1364–9. [DOI] [PubMed] [Google Scholar]
- 8.Miketic S, Carlsson J, Tebbe U. Clinical and angiographic outcome after conventional angioplasty with optional stent implantation compared with direct stenting without predilatation. Heart 2002;88:622–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voigt BJ, Paul M, Pfitzener P, et al. Effectiveness and safety of direct stenting strategy in non-selected lesions. J Am Coll Cardiol 2000;35 (suppl 30):140A. [Google Scholar]
- 10.Chevalier B, Stables R, Te Riele J, et al. Safety and feasibility of direct stenting strategy with the ACS Multi-link Duet stent: results from the SLIDE randomized trial. Circulation 2000;102 (II-730):3529. [Google Scholar]
- 11.Kurki TS, Kataja M, Reich DL. Emergency and elective coronary artery bypass grafting: comparisons of risk profiles, postoperative outcomes, and resource requirements. J Cardiothorac Vasc Anesth 2003;17:594–7. [DOI] [PubMed] [Google Scholar]