Abstract
Objectives: To compare the long term prognosis of patients having silent versus symptomatic ischaemia during dobutamine stress echocardiography (DSE).
Design: Observational study.
Setting: Tertiary referral centre.
Patients: 931 patients who experienced stress induced myocardial ischaemia during DSE.
Results: Silent ischaemia was present in 643 of 931 patients (69%). The number of dysfunctional segments at rest (mean (SD) 9.6 (5.1) v 8.8 (5.0), p = 0.1) and of ischaemic segments (3.5 (2.2) v 3.8 (2.1), p = 0.2) was comparable in both groups. During a mean (SD) follow up of 5.5 (3.3) years, there were 169 (18%) cardiac deaths and 86 (9%) non-fatal infarctions. Multivariable Cox regression analysis showed age (hazard ratio (HR) 1.1, 95% confidence interval (CI) 1.02 to 1.05), previous myocardial infarction (HR 1.4, 95% CI 1.1 to 2.0), and number of ischaemic segments during the test (HR 2.0, 95% CI 1.0 to 3.7) as independent predictors of cardiac death and myocardial infarction. For every additional ischaemic segment there was a twofold increment in risk of late cardiac events. The annual cardiac death or myocardial infarction rate was 3.0% in patients with symptomatic ischaemia and 4.6% in patients with silent ischaemia (p < 0.01). Silent induced ischaemia was an independent predictor of cardiac death and myocardial infarction (HR 1.7, 95% CI 1.1 to 2.0). During follow up symptomatic patients were treated more often with cardioprotective therapy (p < 0.01) and coronary revascularisation (145 of 288 (50%) v 174 of 643 (27%), p < 0.001).
Conclusions: Patients with silent ischaemia had a similar extent of myocardial ischaemia during DSE compared to patients with symptomatic ischaemia but received less cardioprotective treatment and coronary revascularisation and experienced a higher cardiac event rate.
Keywords: silent ischaemia, outcome, dobutamine stress echocardiography
Although angina pectoris is one of the cardinal manifestations of myocardial ischaemia, many patients have ischaemia during stress testing without associated symptoms.1–3 Studies of stress induced silent ischaemia reported discordant results with respect to the extent of ischaemia. Some studies reported a similar extent of ischaemia in patients with and without angina,4,5,6,7,8,9,10 whereas others reported more extensive ischaemia in the presence of angina.11–16 Dobutamine–atropine stress echocardiography (DSE) is commonly used to assess the extent, location, and severity of coronary artery disease.17,18 The diagnosis of myocardial ischaemia during DSE is based on the detection of new or worsening wall motion abnormalities. The extent of these abnormalities is a powerful predictor of adverse outcome.19,20 A large number of studies have reported discordant data regarding the prognostic importance of stress induced silent ischaemia, the likelihood of future coronary events related to the amount of ischaemic myocardium, and the influence of medical treatment or revascularisation techniques on the outcome of silent stress induced ischaemia. Accordingly, the objective of this study was to compare the long term prognosis of silent versus symptomatic ischaemia in a large group of patients undergoing DSE.
PATIENTS AND METHODS
Patient selection
Between 1990 and 2002, 949 consecutive patients experienced stress induced myocardial ischaemia during DSE. Follow up was successful in 944 patients (99.5%). Thirteen patients underwent early coronary revascularisation in the first 60 days after DSE and were excluded from the analysis.21 Data from the remaining 931 patients are reported. The protocol was approved by the hospital ethics committee. All patients gave informed consent before the test. A structured interview and clinical history were taken and cardiac risk factors were assessed before DSE.
Dobutamine stress protocol
Dobutamine–atropine stress testing was performed according to a standard protocol as previously reported.19,22,23 Dobutamine was administered intravenously, starting at a dose of 5 μg/kg/min for five minutes, followed by 10 μg/kg/min for five minutes. Subsequently, incremental dobutamine doses of 10 μg/kg/min were given at three minute intervals up to a maximum dose of 40 μg/kg/min. If the test end point was not reached at a dobutamine dose of 40 μg/kg/min, atropine (up to 2 mg) was given intravenously. Blood pressure, heart rate, and ECG were constantly monitored. Test end points were achievement of the target heart rate (85% of maximum age and sex predicted heart rate), maximum dose of dobutamine and atropine, horizontal or downsloping ST segment depression > 2 mm at an interval of 80 ms after the J point compared with baseline, severe angina, systolic blood pressure fall > 40 mm Hg, blood pressure > 240/120 mm Hg, or significant cardiac arrhythmia. An intravenous β blocker was available to reverse the adverse effects of dobutamine and atropine.
Stress echocardiography
Two dimensional echocardiographic images were acquired at rest, during dobutamine stress, and during recovery. The echocardiograms were recorded in a quad screen format. Two experienced observers, unaware of the clinical data, scored the echocardiograms according to a standard 16 segment model. Regional wall motion and systolic wall thickening were scored on a five point scale (1 indicating normal; 2, mild hypokinesia; 3, severe hypokinesia; 4, akinesia; and 5, dyskinesia). Ischaemia was defined as new or worsened wall motion abnormalities during stress indicated by an increase of wall motion score ⩾ 1 grade in ⩾ 1 segments. Ischaemia was not considered to be present when akinetic segments at rest became dyskinetic during stress. Symptomatic ischaemia was defined as the presence of typical chest pain or anginal equivalent symptoms (epigastric pain, jaw pain, and dyspnoea) during DSE. Coronary arteries were assigned to myocardial segments based on echocardiographic localisation, as previously described.24 The anterior, apical, septal, and anteroseptal walls were assigned to the left anterior descending coronary artery, the posterior and lateral wall to the left circumflex, and the inferior and basal septal segments to the right coronary artery. The apical lateral segment was considered to be an overlapping segment between the left anterior descending coronary artery and the left circumflex, and the apical inferior segment was considered to be an overlapping segment between the left anterior descending coronary artery and the right coronary artery. Overlapping segments were assigned to the regions with concomitant abnormalities.
Follow up
Follow up data were collected by contacting the patient’s general practitioner and by review of hospital records. The date of the last review or consultation was used to calculate follow up time. Outcome events were overall death, cardiac death, and non-fatal myocardial infarction. Cardiac death was defined as death caused by acute myocardial infarction, significant cardiac arrhythmias, or refractory congestive heart failure. Sudden death occurring without another explanation was regarded as cardiac death. Non-fatal myocardial infarction was defined by two of the following symptoms: typical chest pain, increased cardiac enzyme concentrations, and typical changes on ECG.
Statistical analysis
Continuous data were expressed as mean (SD). Student’s t test was used to analyse continuous data. Differences between proportions were compared with the χ2 test. Univariate and multivariate Cox proportional hazard regression models (BMDP Statistical Software Inc, Los Angeles, California, USA) were used to identify independent predictors of late cardiac events.25 Variables were selected in a stepwise forward selection manner, including clinical and DSE data, with entry and retention set at a significance level of 0.05. The risk of a variable was expressed as a hazard ratio (HR) with a corresponding 95% confidence interval (CI). The probability of survival was calculated by the Kaplan-Meier method and survival curves were compared by the log rank test. A probability value of p < 0.05 was considered significant.
RESULTS
Patient characteristics and haemodynamic results
Silent myocardial ischaemia was present in 643 of 931 (69%) patients. Clinical risk factors were not significantly different between patients with and patients without angina during DSE, with the exception of history of angina, which was more often observed in patients with symptomatic ischaemia (table 1). Table 2 shows DSE characteristics of patients with silent and symptomatic myocardial ischaemia. There was no significant difference between patients with and patients without silent ischaemia with respect to the number of abnormal segments at rest and the number of ischaemic segments during DSE. No patient experienced a myocardial infarction during the test. Side effects among the 931 patients were non-sustained ventricular tachycardia (< 10 complexes) in 28 (3%), sustained ventricular tachycardia (> 10 complexes) in 13 (1%), severe hypotension (decrease in systolic blood pressure > 40 mm Hg compared with baseline) in seven (1%), atrial fibrillation in six (1%), and ventricular fibrillation in one (0.1%).
Table 1.
Clinical characteristics of patients with and without angina during dobutamine stress echocardiography (DSE)
| Silent ischaemia (n = 643) | Symptomatic ischaemia (n = 288) | p Value | |
| Age (years)* | 61 (13) | 61 (11) | 0.6 |
| Men | 465 (72%) | 233 (81%) | 0.009 |
| Previous infarction | 330 (51%) | 161 (56%) | 0.2 |
| Diabetes mellitus | 78 (12%) | 29 (10%) | 0.4 |
| Hypertension | 195 (30%) | 79 (27%) | 0.4 |
| Hypercholesterolaemia | 163 (25%) | 82 (28%) | 0.3 |
| Smoking | 122 (19%) | 88 (30%) | 0.3 |
| History of angina | 200 (31%) | 196 (68%) | <0.001 |
| History of heart failure | 107 (17%) | 38 (13%) | 0.2 |
| Previous coronary angiography | 219 (34%) | 112 (39%) | 0.2 |
| Previous coronary bypass surgery | 96 (15%) | 43 (15%) | 1 |
| Previous coronary angioplasty | 89 (14%) | 56 (19%) | 0.04 |
| β Blockers | 243 (38%) | 115 (40%) | 0.5 |
| Calcium channel blockers | 177 (27%) | 99 (34%) | 0.04 |
| Nitrates | 180 (28%) | 115 (40%) | 0.001 |
| Aspirin | 302 (47%) | 158 (55%) | 0.005 |
| Statins | 174 (27%) | 92 (32%) | 0.01 |
| Indication for DSE | |||
| Diagnosis of CAD | 350 (54%) | 211 (73%) | <0.0001 |
| Preoperative evaluation before non-cardiac surgery | 186 (29%) | 34 (12%) | <0.0001 |
| Risk stratification after MI | 107 (17%) | 43 (15%) | 0.6 |
*Mean (SD).
CAD, coronary artery disease; MI, myocardial infarction.
Table 2.
DSE data for patients with and without angina during DSE
| Silent ischaemia (n = 643) | Symptomatic ischaemia (n = 288) | p Value | |
| Peak heart rate (beats/min) | 127 (21) | 124 (20) | 0.03 |
| Peak systolic blood pressure (mm Hg) | 135 (31) | 134 (28) | 0.7 |
| Peak rate pressure product | 16 573 (4290) | 17 119 (4767) | 0.1 |
| Peak dobutamine dose (μg) | 37 (7) | 38 (5) | 0.09 |
| Atropine use | 230 (36%) | 96 (33%) | 0.5 |
| ST segment depression | 248 (38%) | 179 (62%) | <0.001 |
| Number of dysfunctional segments at rest | 9.6 (5.1) | 8.8 (5.0) | 0.1 |
| Number of ischaemic segments | 3.5 (2.2) | 3.8 (2.1) | 0.2 |
| Arrhythmias and hypotension during the test | 43 (7%) | 12 (4%) | 0.2 |
| Reasons for termination of the stress test | |||
| 85% of maximum heart rate | 579 (90%) | 250 (87%) | 0.2 |
| Maximum dose | 36 (5%) | 14 (5%) | 0.8 |
| Arrhythmias | 12 (2%) | 2 (1%) | 0.3 |
| Side effects | 11 (2%) | 1 (0.3%) | 0.2 |
| ST segment depression | 5 (1%) | 10 (3%) | <0.005 |
| Angina | 0 (0%) | 11 (4%) | <0.0001 |
Data are mean (SD) or number (%).
DSE and outcome
During a mean (SD) 5.5 (3.3) years follow up of the 931 patients, there were 265 (28%) deaths, of which 169 (18%) were attributed to cardiac causes. Non-fatal infarction occurred in 86 (9%) patients and late revascularisation was performed in 319 (34%) patients. The annual cardiac death rate was 2.2% in the group of patients with symptomatic ischaemia during DSE and 3.8% in the group with silent ischaemia (p < 0.01). The annual myocardial infarction or cardiac death rate was 3.0% in patients with symptomatic ischaemia and 4.6% in patients with silent ischaemia (p < 0.01). Late revascularisation was more often performed in patients with symptomatic ischaemia (145 of 288 (50%) v 174 of 643 (27%), p < 0.001). Medical treatment was changed after detection of ischaemia by DSE in both groups but patients with angina during DSE were significantly more often treated with β blockers, aspirin, and statins (table 3). On the other hand, the fact that fewer of these patients were treated with nitrates and calcium channel blockers at follow up is related to the higher incidence of coronary revascularisation in this group.
Table 3.
Medication at follow up of patients with and without angina during DSE
| Baseline | Follow up | |||||
| Silent ischaemia (n = 643) | Symptomatic ischaemia (n = 288) | p Value | Silent ischaemia (n = 643) | Symptomatic ischaemia (n = 288) | p Value | |
| β blockers | 243 (38%) | 115 (40%) | 0.5 | 276 (43%) | 202 (70%) | <0.001 |
| Calcium channel blockers | 177 (27%) | 99 (34%) | 0.04 | 193 (30%) | 89 (31%) | 0.06 |
| Nitrates | 180 (28%) | 115 (40%) | 0.001 | 193 (30%) | 69 (24%) | <0.006 |
| Aspirin | 302 (47%) | 158 (55%) | 0.005 | 334 (52%) | 236 (82%) | <0.001 |
| Statins | 174 (27%) | 92 (32%) | 0.1 | 263 (41%) | 245 (85%) | <0.001 |
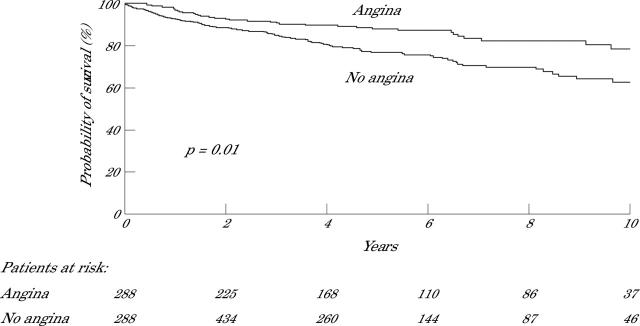
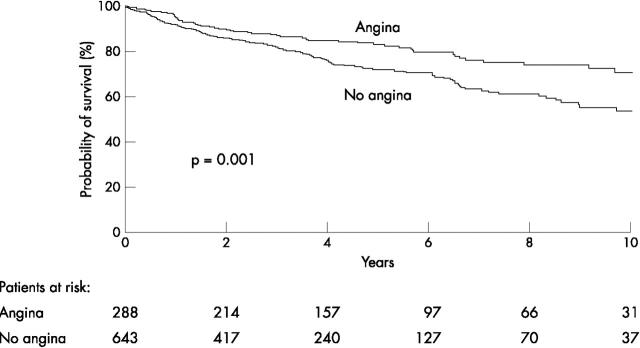
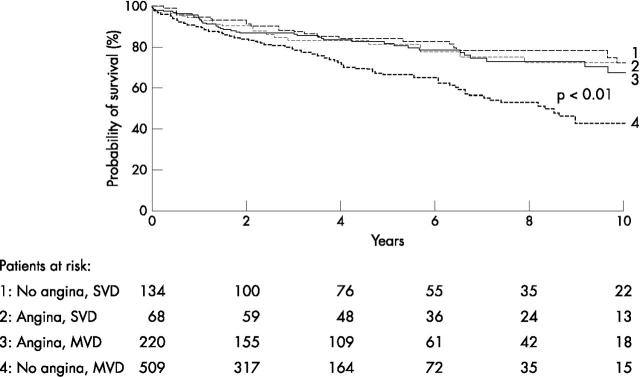
Figures 1 and 2 present Kaplan-Meier survival curves for the end point cardiac death and the combined end point cardiac death or non-fatal infarction, respectively. Patients with silent ischaemia had a significantly lower probability of survival during long term follow up than patients with symptomatic ischaemia. In particular, patients with silent ischaemia and multivessel disease had a poor long term prognosis (fig 3). This may be related to the fact that patients with symptomatic ischaemia and multivessel disease were more often referred for coronary revascularisation than patients with silent ischaemia and multivessel disease (111 of 220 (50%) v 141 of 509 (28%), p < 0.0001). Among patients with symptomatic ischaemia and multivessel disease, cardiac death occurred in 11 of 111 (11%) patients who were revascularised versus 22 of 109 (20%) patients who were treated medically (p = 0.05). Similarly, in patients with silent ischaemia and multivessel disease, cardiac death occurred in 18 of 141 (13%) patients who were revascularised versus 92 of 368 (25%) medically treated patients (p = 0.004).
Figure 1.
Kaplan-Meier survival curves (end point of cardiac death) for patients with silent versus patients with symptomatic ischaemia during dobutamine stress echocardiography (DSE).
Figure 2.
Kaplan-Meier survival curves (end point of cardiac death and non-fatal myocardial infarction) for patients with silent versus patients with symptomatic ischaemia during DSE.
Figure 3.
Kaplan-Meier survival curves showing cardiac event rate in patients with (1) silent ischaemia and single vessel disease (SVD); (2) symptomatic ischaemia and single vessel disease; (3) symptomatic ischaemia and multivessel disease (MVD); and (4) silent ischaemia and multivessel disease.
No sex differences for cardiac death or hard cardiac events were present in the overall population, in the two groups of patients with symptomatic or silent ischaemia, or in the two groups of patients with single or multivessel disease.
Incremental prognostic value
Independent predictors of cardiac death in a multivariate analysis were age (HR 1.1, 95% CI 1.04 to 1.08), male sex (HR 1.8, 95% CI 1.2 to 2.7), smoking (HR 1.5, 95% CI 1.1 to 2.0), and the number of ischaemic segments during DSE (HR 2.1, 95% CI 1.1 to 4.1). Independent predictors of cardiac death or myocardial infarction were age (HR 1.1, 95% CI 1.02 to 1.05), previous myocardial infarction (HR 1.4, 95% CI 1.1 to 2.0), and the number of ischaemic segments during DSE (HR 2.0, 95% CI 1.0 to 3.7). For every ischaemic segment there was a twofold increment in risk of late cardiac events. Silent induced ischaemia was also an independent predictor of cardiac death and myocardial infarction (HR 1.7, 95% CI 1.1 to 2.0). An interaction term between silent induced ischaemia and the presence of ischaemia was not significant.
DISCUSSION
The current results show that patients with silent myocardial ischaemia during DSE have a higher incidence of long term cardiac death and myocardial infarction than do patients with symptomatic myocardial ischaemia. This association was observed irrespective of the presence and extent of resting or dobutamine stress induced new wall motion abnormalities.
Significant changes were observed in medical treatment of patients with and without angina during DSE. Patients with silent myocardial ischaemia during DSE were less often prescribed β blockers, aspirin, and statins than were patients with symptomatic ischaemia. In addition, late revascularisation was less often performed in patients with asymptomatic ischaemia during DSE. Moreover, in patients with multivessel disease and symptomatic or silent ischaemia during DSE, cardiac death occurred more often in medically treated patients than in patients who underwent myocardial revascularisation.
The worse long term prognosis of patients with asymptomatic myocardial ischaemia may be associated with less aggressive anti-ischaemic medical treatment and coronary revascularisation. β Blockers have been unequivocally shown in multiple studies to reduce angina and the incidence, frequency, and extension of episodes of silent ischaemia.26–28 The combination of β blockers with other anti-ischaemic medical treatment such as calcium channel antagonists has also been found to reduce the incidence of myocardial ischaemia more than either type of these medications alone.29,30 Also, statins have been shown to decrease transient myocardial ischaemia, probably due to improved endothelial function.31 Furthermore, myocardial revascularisation was previously shown in a prospective study to be associated with decreased asymptomatic ischaemia and improved clinical outcome compared with angina or ischaemia guided strategy.32 In the present study, the decision to perform coronary angiography was made on clinical grounds by the treating cardiologist. The prescription rate of β blockers or calcium antagonist was relatively low. It seems that in some patients symptoms of angina instead of signs of ischaemia on DSE were the reason to prescribe β blockers or calcium antagonists and to perform myocardial revascularisation.
Additionally, in this study absence of symptoms during dobutamine stress induced ischaemia was often observed (69%); this is in line with previous studies4,33,34 and is physiologically explained by the ischaemic cascade, since systolic dysfunction precedes the development of angina pectoris.
Comparison with previous studies
There are only a few studies on the long term prognostic implications of silent myocardial ischaemia during DSE. Bigi and colleagues35 studied 407 survivors from a first uncomplicated myocardial infarction who had myocardial ischaemia during DSE and were followed up for a 10 month period. Cardiac death and non-fatal myocardial re-infarction occurred, respectively, in six of 407 (1%) and 13 of 407 (3%) patients. No significant difference in spontaneous event-free survival was observed among patients with silent ischaemia. Bonou and colleagues33 studied 289 patients with advanced age undergoing DSE. During a 35 (13) month follow up period, 15 (5%) cardiac deaths and 19 (7%) non-fatal myocardial infarctions occurred. There was no significant difference in prognosis between patients with silent ischaemia and patients with symptomatic myocardial ischaemia. In the present study the cardiac event rate was higher than in the previous studies; this may be related to the longer and nearly complete (99.5%) follow up. Previous data from 224 patients who underwent dobutamine stress myocardial perfusion imaging showed a similar outcome between patients with silent ischaemia and patients with symptomatic ischaemia.34 The different findings in that study may be related to differences in sensitivity between myocardial perfusion imaging and DSE, as well as a possible differences in management of patients after a positive study by these two techniques.
Some previous studies suggested a common pathway for both the electrical and pain response to ischaemic stimulus.8 These studies showed that during exercise stress testing patients with symptomatic ischaemia had a higher incidence of ST segment changes.8,14,15 However, the extent of ischaemia was comparable between patients with and patients without symptomatic ischaemia during DSE. The mechanism underlying the pathogenesis of silent or symptomatic ischaemia is complex and may include variations in pain threshold, a central nervous system alteration, or a particular biochemical pattern of inflammatory system activation.36,37 The relation between angina and extent of ischaemia is not clear. Several studies have assessed the extent of ischaemia in the presence and in the absence of symptomatic myocardial ischaemia during stress test with contradictory results. Some investigators have reported a greater extent and severity of ischaemia in symptomatic patients than in those with silent myocardial ischaemia,11–16 whereas other studies reported no difference in the amount of ischaemic myocardium between patients with and patients without symptomatic myocardial ischaemia during exercise or pharmacological stress testing.4,5,6,7,8,9,10 In the present study there are no significant differences between dysfunctional segments at rest and the number of ischaemic segments at peak in patients with and without symptomatic myocardial ischaemia. Moreover, the extent of ischaemia during DSE was an independent predictor of cardiac death and myocardial infarction, and the number of diseased vessels was related to an increased rate of cardiac events, particularly in patients with symptomatic ischaemia. This is in line with previous studies that show the total amount of ischaemic territory at risk is related more to the outcome than to the presence of symptoms.3,18,19,38,39
Study limitations
Data on coronary angiography were not available for all patients.
Conclusion
This study showed that patients with asymptomatic myocardial ischaemia during DSE had a worse long term cardiac event-free survival rate than did patients with symptomatic myocardial ischaemia. Patients with asymptomatic ischaemia should be treated with a complete medical therapy or revascularisation as patients with symptomatic myocardial ischaemia.
REFERENCES
- 1.Laukkanen JA, Kurl S, Lakka TA, et al. Exercise-induced silent myocardial ischemia and coronary morbidity and mortality in middle-aged men. J Am Coll Cardiol 2001;38:72–9. [DOI] [PubMed] [Google Scholar]
- 2.Almeda FQ, Kason TT, Nathan S, et al. Silent myocardial ischemia: concepts and controversies. Am J Med 2004;116:112–8. [DOI] [PubMed] [Google Scholar]
- 3.Miranda CP, Lehmann KG, Lachterman B, et al. Comparison of silent and symptomatic ischemia during exercise testing in men. Ann Intern Med 1991;114:649–56. [DOI] [PubMed] [Google Scholar]
- 4.Bolognese L, Rossi L, Sarasso G, et al. Silent versus symptomatic dipyridamole-induced ischemia after myocardial infarction: clinical and prognostic significance. J Am Coll Cardiol 1992;19:953–9. [DOI] [PubMed] [Google Scholar]
- 5.Mahmarian JJ, Pratt CM, Cocanougher MK, et al. Altered myocardial perfusion in patients with angina pectoris or silent ischemia during exercise as assessed by quantitative thallium-201 single-photon emission computed tomography. Circulation 1990;82:1305–15. [DOI] [PubMed] [Google Scholar]
- 6.Hecht HS, DeBord L, Sotomayor N, et al. Truly silent ischemia and the relationship of chest pain and ST segment changes to the amount of ischemic myocardium: evaluation by supine bicycle stress echocardiography. J Am Coll Cardiol 1994;23:369–76. [DOI] [PubMed] [Google Scholar]
- 7.Gasperetti CM, Burwell LR, Beller GA. Prevalence of and variables associated with silent myocardial ischemia on exercise thallium-201 stress testing. J Am Coll Cardiol 1990;16:115–23. [DOI] [PubMed] [Google Scholar]
- 8.Hecht HS, Shaw RE, Bruce T, et al. Silent ischemia: evaluation by exercise and redistribution tomographic thallium-201 myocardial imaging. J Am Coll Cardiol 1989;14:895–900. [DOI] [PubMed] [Google Scholar]
- 9.Marwick TH, Nemec JJ, Torelli J, et al. Extent and severity of abnormal left ventricular wall motion detected by exercise echocardiography during painful and silent ischemia. Am J Cardiol 1992;69:1483–4. [DOI] [PubMed] [Google Scholar]
- 10.Mark DB, Hlatky MA, Califf RM, et al. Painless exercise ST deviation on the treadmill: long-term prognosis. J Am Coll Cardiol 1989;14:885–92. [DOI] [PubMed] [Google Scholar]
- 11.Marwick TH, Case C, Short L, et al. Prediction of mortality in patients without angina: use of an exercise score and exercise echocardiography. Eur Heart J 2003;24:1223–30. [DOI] [PubMed] [Google Scholar]
- 12.Nihoyannopoulos P, Marsonis A, Joshi J, et al. Magnitude of myocardial dysfunction is greater in painful than in painless myocardial ischemia: an exercise echocardiographic study. J Am Coll Cardiol 1995;25:1507–12. [DOI] [PubMed] [Google Scholar]
- 13.Narins CR, Zareba W, Moss AJ, et al. Clinical implications of silent versus symptomatic exercise-induced myocardial ischemia in patients with stable coronary disease. J Am Coll Cardiol 1997;29:756–63. [DOI] [PubMed] [Google Scholar]
- 14.Klein J, Chao SY, Berman DS, et al. Is ‘silent’ myocardial ischemia really as severe as symptomatic ischemia? The analytical effect of patient selection biases. Circulation 1994;89:1958–66. [DOI] [PubMed] [Google Scholar]
- 15.Travin MI, Flores AR, Boucher CA, et al. Silent versus symptomatic ischemia during a thallium-201 exercise test. Am J Cardiol 1991;68:1600–8. [DOI] [PubMed] [Google Scholar]
- 16.Marcassa C, Galli M, Baroffio C, et al. Ischemic burden in silent and painful myocardial ischemia: a quantitative exercise sestamibi tomographic study. J Am Coll Cardiol 1997;29:948–54. [DOI] [PubMed] [Google Scholar]
- 17.Segar DS, Brown SE, Sawada SG, et al. Dobutamine stress echocardiography: correlation with coronary lesion severity as determined by quantitative angiography. J Am Coll Cardiol 1992;19:1197–202. [DOI] [PubMed] [Google Scholar]
- 18.Lancellotti P, Benoit T, Rigo P, et al. Dobutamine stress echocardiography versus quantitative technetium-99m sestamibi SPECT for detecting residual stenosis and multivessel disease after myocardial infarction. Heart 2001;86:510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schinkel AF, Bax JJ, Geleijnse ML, et al. Noninvasive evaluation of ischaemic heart disease: myocardial perfusion imaging or stress echocardiography? Eur Heart J 2003;24:789–800. [DOI] [PubMed] [Google Scholar]
- 20.Sicari R, Pasanisi E, Venneri L, et al. Stress echo results predict mortality: a large-scale multicenter prospective international study. J Am Coll Cardiol 2003;41:589–95. [DOI] [PubMed] [Google Scholar]
- 21.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation 1996;93:905–14. [DOI] [PubMed] [Google Scholar]
- 22.Elhendy A, van Domburg RT, Bax JJ, et al. The grade of worsening of regional function during dobutamine stress echocardiography predicts the extent of myocardial perfusion abnormalities. Heart 2000;83:35–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann R, Marwick TH, Poldermans D, et al. Refinements in stress echocardiographic techniques improve inter-institutional agreement in interpretation of dobutamine stress echocardiograms. Eur Heart J 2002;23:821–9. [DOI] [PubMed] [Google Scholar]
- 24.Marwick TH, D’Hondt AM, Baudhuin T, et al. Optimal use of dobutamine stress for the detection and evaluation of coronary artery disease: combination with echocardiography, scintigraphy or both? J Am Coll Cardiol 1993;22:159–67. [DOI] [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life-tables. J R Stat Soc (B) 1972;34:187–202. [Google Scholar]
- 26.Egstrup K. Randomized double-blind comparison of metoprolol, nifedipine, and their combination in chronic stable angina: effects on total ischemic activity and heart rate at onset of ischemia. Am Heart J 1988;116:971–8. [DOI] [PubMed] [Google Scholar]
- 27.Stone PH, Gibson RS, Glasser SP, et al. Comparison of propranolol, diltiazem, and nifedipine in the treatment of ambulatory ischemia in patients with stable angina: differential effects on ambulatory ischemia, exercise performance, and anginal symptoms. The ASIS study group. Circulation 1990;82:1962–72. [DOI] [PubMed] [Google Scholar]
- 28.Quyyumi AA, Crake T, Wright CM, et al. Medical treatment of patients with severe exertional and rest angina: double blind comparison of beta blocker, calcium antagonist, and nitrate. Br Heart J 1987;57:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies RF, Habibi H, Klinke WP, et al. Effect of amlodipine, atenolol and their combination on myocardial ischemia during treadmill exercise and ambulatory monitoring. Canadian amlodipine/atenolol in silent ischemia study (CASIS) investigators. J Am Coll Cardiol 1995;25:619–25. [DOI] [PubMed] [Google Scholar]
- 30.Resnekov L. Silent myocardial ischemia: therapeutic implications. Am J Med 1985;79:30–4. [DOI] [PubMed] [Google Scholar]
- 31.Aengevaeren WR, Uijen GJ, Jukema JW, et al. Functional evaluation of lipid-lowering therapy by pravastatin in the regression growth evaluation statin study (REGRESS). Circulation 1997;96:429–35. [DOI] [PubMed] [Google Scholar]
- 32.Knatterud GL, Bourassa MG, Pepine CJ, et al. Effects of treatment strategies to suppress ischemia in patients with coronary artery disease: 12-week results of the asymptomatic cardiac ischemia pilot (ACIP) study. J Am Coll Cardiol 1994;24:11–20. [DOI] [PubMed] [Google Scholar]
- 33.Bonou M, Benroubis A, Kranidis A, et al. Functional and prognostic significance of silent ischemia during dobutamine stress echocardiography in the elderly. Coron Artery Dis 2001;12:499–506. [DOI] [PubMed] [Google Scholar]
- 34.Elhendy A, Schinkel AFL, van Domburg RT, et al. Comparison of late outcome in patients with versus without angina pectoris having reversible perfusion abnormalities during dobutamine stress technetium-99m sestamibi single-photon emission computed tomography. Am J Cardiol 2003;91:264–8. [DOI] [PubMed] [Google Scholar]
- 35.Bigi R, Galati A, Curti G, et al. Different clinical and prognostic significance of painful and silent myocardial ischemia detected by exercise electrocardiography and DSE after uncomplicated myocardial infarction. Am J Cardiol 1998;81:75–8. [DOI] [PubMed] [Google Scholar]
- 36.Mazzone A, Cusa C, Mazzucchelli I, et al. Increased production of inflammatory cytokines in patients with silent myocardial ischemia. J Am Coll Cardiol 2001;38:1895–901. [DOI] [PubMed] [Google Scholar]
- 37.Rosen SD, Paulesu E, Nihoyannopoulos P, et al. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med 1996;124:939–49. [DOI] [PubMed] [Google Scholar]
- 38.Deedwania PC, Carbajal EV. Silent myocardial ischemia: a clinical perspective. Arch Intern Med 1991;151:2373–82. [PubMed] [Google Scholar]
- 39.Weiner DA, Ryan TJ, McCabe CH, et al. Comparison of coronary artery bypass surgery and medical therapy in patients with exercised-induced silent myocardial ischemia: a report from the coronary artery surgery study (CASS) registry. J Am Coll Cardiol 1988;12:595–9. [DOI] [PubMed] [Google Scholar]