Abstract
Objectives: To investigate the characteristics of the acute coronary syndromes underlying acute pulmonary oedema and their 30 day prognosis.
Patients: 185 consecutive patients with acute coronary syndromes and acute pulmonary oedema admitted to a tertiary care centre.
Main outcome and measures: Clinical, ECG, echocardiographic, enzymatic, and angiographic features were prospectively investigated.
Results: Non-ST segment elevation myocardial infarction (NSTEMI) was the most frequent cause of acute pulmonary oedema (61%) followed by unstable angina (UA; 21%) and ST segment elevation myocardial infarction (STEMI; 18%). In each group, mean age was ⩾ 70 years, but NSTEMI patients were the oldest and ⩾ 65% of patients had chronic hypertension. Moreover, patients with NSTEMI and UA were older and had a higher incidence of diabetes, previous myocardial infarction, and moderate to severe mitral regurgitation but a similarly reduced ejection fraction (NSTEMI, 41%; UA, 39%; and STEMI, 39%) and increased incidence of diastolic dysfunction and rate of multivessel disease (94%, 87%, and 86%, respectively). However, patients with STEMI had a higher creatine kinase MB peak concentration (158 v 76 μg/l in the NSTEMI group, p < 0.001) and 30 day mortality (26% v 9% in the NSTEMI group and 8% in the UA group, p < 0.024). Multivariate analysis identified ejection fraction < 40% and a peak creatine kinase MB concentration > 100 μg/l as the main prognostic markers (p < 0.03).
Conclusions: Acute pulmonary oedema is mostly a complication of elderly hypertensive patients with NSTEMI or UA (82%) and with multivessel disease often associated with mitral regurgitation. On the other hand, the larger infarct size and higher mortality in patients with STEMI with a similarly reduced ejection fraction suggest a more extensive acute systolic loss.
Keywords: NSTEMI, prognosis, STEMI, unstable angina, acute pulmonary oedema
Clinical manifestations of acute cardiogenic pulmonary oedema appear to be independent of the kind of underlying acute coronary syndromes (ACS),1–8 whether ST segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI), or unstable angina (UA). However, the proportion of each of these ACS among consecutive patients with acute pulmonary oedema and their 30 day prognosis remains to be defined. Thus, we elected to investigate prospectively the frequency of each ACS in patients with acute pulmonary oedema and their clinical, ECG, echocardiographic, and angiographic features, as well as their 30 day mortality. In addition, even though acute pulmonary oedema is largely a complication of the elderly,1,9,10 we assessed the relevance of other contributing factors by comparing the features of patients with ACS ⩾ 70 years with and without acute pulmonary oedema.
METHODS
Patients
Of a total of 216 consecutive patients admitted to our emergency room with acute pulmonary oedema between February 2000 and December 2001, 185 with ACS were enrolled in this prospective study. Acute pulmonary oedema was defined by the presence of frank orthopnoea of sudden onset (⩽ 6 hours), inspiratory rales, documented hypoxaemia (arterial oxygen saturation < 90%, with or without oxygen supply), and alveolar or interstitial pulmonary oedema on chest radiography. Patients with chronic heart failure who presented a more gradual aggravation, however, were excluded. Diagnosis of STEMI was based on the presence of ST elevation with or without chest pain associated with an acute enzyme rise more than twice the upper normal concentration (creatine kinase (CK) MB mass > 10 μg/l). Patients with increased enzymes but without ST elevation were categorised as having NSTEMI. Patients with myocardial infarction (MI) and left bundle branch block (LBBB) were considered to have NSTEMI except for patients with acute LBBB. Diagnosis of UA was based on the presence of angina at rest or at minimum exertion with transient repolarisation changes in the ECG and no enzyme rise. To analyse the possible role of factors other than advanced age as relevant contributors to acute pulmonary oedema, the characteristics of patients with an acute pulmonary oedema who were ⩾ 70 years old were compared with characteristics of 74 other patients admitted consecutively during the same interval with ACS and ⩾ 70 years old but without acute pulmonary oedema.
Protocol
Digital pulse oxymetry, arterial blood gas, or both were determined on admission and repeated subsequently according to clinical course. A standard 12 lead ECG was also recorded on admission and repeated at least once after clinical stabilisation or on deterioration. Shortly after admission a chest radiograph was recorded for all patients and blood was sampled for CKMB mass and cardiac troponin I determination and repeated every six hours, at least during the first 24 hours. Patients were treated with oxygen by mask, morphine sulphate, and intravenous infusion of glyceryl trinitrate and furosemide. In cases of persistent arterial hypertension sodium nitroprusside was added. Hypotension was treated with dobutamine or dopamine. Aspirin and subcutaneous low molecular weight heparin were also administered. Patients with persistent respiratory insufficiency, particularly when associated with carbon dioxide retention, received ventilatory support with continuous positive airway pressure. Oral intubation and mechanical ventilation were instituted for patients with refractory hypoventilation.
Echocardiographic study
A transthoracic two dimensional echocardiogram (CFM 750; Vingmed, Horten, Norway) was recorded for all patients, for 107 (58%) during the first 36 hours and for the rest during the first week. Thickness of septal and posterior walls was measured in the long or short parasternal views by M mode. Ejection fraction was measured by Simpson’s method and segmental wall motion was qualitatively evaluated as normal, hypokinetic, or akinetic/dyskinetic. A dilated left ventricle was considered when end diastolic diameter was > 60 mm. The presence of valvar disease was also investigated and particular attention was paid to the existence of mitral regurgitation (MR), which was assessed by a semiquantitative approach by Doppler flow mapping (pulsed or colour). MR was categorised as mild, moderate, or severe when the regurgitant jet occupied 5–19%, 20–39%, or ⩾ 40%, respectively, of the left atrial area.9 Diastolic transmitral flow velocities were recorded in the standard apical four chamber view with the sample volume positioned at the mitral leaflet tips. The ratio between peak velocities of the E and A waves was evaluated and the diastolic inflow was categorised as having normal, impaired relaxation, pseudonormal, or restrictive patterns.10
Coronary angiography
Coronary angiography was performed in 99 patients (53%) and the reasons that prevented its practice in the remaining 86 were early in-hospital death (seven (8%)), age > 80 years (33 (38%)) of whom nearly half had additional co-morbidity factors, a recent cerebrovascular accident (four (5%)), senile dementia (seven (8%)), severe chronic lung disease (four (5%)), chronic renal failure (nine (10%)), malignant neoplasm (11 (13%)), patient’s refusal (eight (9%)), or attending physician’s decision (eight (9%)). Moreover, patients without angiography were older than those who did undergo catheterisation (80 (9) v 70 (10) years, p < 0.001) and had a higher incidence diabetes mellitus (54% v 35%, p < 0.004).
All procedures were performed according to institutional guidelines and patients provided informed consent before entering the study.
Statistical analysis
The χ2 test or the Fisher exact test were used to compare categorical variables. Student’s t test was used to assess intergroup differences of continuous variables. A multivariate analysis of variables related to in-hospital mortality was used to assess variables with significantly higher incidence in non-survivors than in survivors. The analysis was performed with SPSS 10.0 (SPSS Inc, Chicago, Illinois, USA), data were expressed as mean (SD), and differences were considered significant at the level of p < 0.05.
RESULTS
Clinical and ECG data
Thirty four patients had a STEMI (18%), 112 an NSTEMI (61%), and 39 UA (21%). Patients with STEMI had a lower incidence of diabetes mellitus, antecedent acute pulmonary oedema, previous atrial fibrillation, and old MI than those with NSTEMI or UA (table 1), but they had a higher peak CKMB mass and cardiac troponin I concentration. Nevertheless, in an important proportion of patients with MI peak CK was modest at most (< 80 μg/l in 133 (72%) and < 30 μg/l in 102 (55%)). There were 41 patients with LBBB and all were included in the NSTEMI group, since there was no evidence that the conduction abnormality was acute (the existing ECG already showed an LBBB or there were no previous ECGs). However, among patients with LBBB and those with NSTEMI and ST segment depression (n = 51), there was a comparable proportion of men (46% v 53%) and incidences of hypertension (71% v 78%), diabetes (56% v 53%), peripheral vascular disease (46% v 63%), previous MI (42% v 41%), and previous acute pulmonary oedema (34% v 20%).
Table 1.
Demographic data of patients with acute pulmonary oedema (APO) with ST segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infarction (NSTEMI), and unstable angina (UA)
| STEMI (a, n = 34) | NSTEMI (b, n = 112) | UA (c, n = 39) | p Value | |||
| a v b | a v c | b v c | ||||
| Age (years) | 71.4 (12.2) | 77.3 (12.2) | 71.0 (10.4) | <0.01 | NS | <0.01 |
| ⩾70 years | 21 (62%) | 94 (84%) | 23 (59%) | <0.01 | NS | <0.01 |
| Men | 21 (62%) | 58 (52%) | 22 (56%) | NS | NS | NS |
| Hypertension | 22 (65%) | 83 (74%) | 26 (67%) | <0.04 | NS | NS |
| Diabetes mellitus | 13 (38%) | 57 (51%) | 19 (49%) | <0.05 | NS | NS |
| Cholesterol >6 mmol/l | 12 (35%) | 40 (36%) | 12 (31%) | NS | NS | NS |
| PVD | 16 (47%) | 61 (55%) | 18 (47%) | NS | NS | NS |
| Old myocardial infarction | 8 (24%) | 47 (42%) | 21 (54%) | <0.013 | <0.001 | NS |
| Previous atrial fibrillation | 3 (9%) | 29 (26%) | 10 (26%) | <0.012 | <0.017 | NS |
| Previous heart failure | 8 (24%) | 47 (42%) | 22 (56%) | <0.013 | <0.001 | NS |
| Previous APO | 3 (9%) | 28 (25%) | 13 (34%) | <0.015 | <0.002 | NS |
| Peak CKMB mass (μg/l) | 158 (208) | 76 (103) | 4 (2) | <0.001 | <0.001 | <0.001 |
| Peak cTnI (μg/l) | 61.3 (105.2) | 18.8 (23.3) | 0.4 (0.4) | <0.001 | <0.001 | <0.001 |
| Admission | ||||||
| Heart rate (beats/min) | 117 (17) | 113 (23) | 103 (27) | NS | NS | NS |
| Systolic BP (mm Hg) | 155 (47) | 155 (43) | 160 (40) | NS | NS | NS |
| Diastolic BP (mm Hg) | 90 (24) | 85 (24) | 88 (25) | NS | NS | NS |
Data are mean (SD) or number (%).
BP, blood pressure; CK, creatine kinase; cTnI, cardiac troponin I; NS, not significant; PVD, peripheral vascular disease.
Twenty one patients (11%) presented with atrial fibrillation at a rate of > 130 beats/min that was interpreted as a concurrent event or as a possible trigger of acute pulmonary oedema. In the rest there were no apparent triggers other than the ischaemic events.
Echocardiographic and angiographic data
The three groups had a high and similar incidence of left ventricular hypertrophy, reduced ejection fraction (< 40%), and diastolic dysfunction (table 2). Patients with STEMI had a lower rate of moderate to severe MR than those with NSTEMI. However, the incidence of multivessel disease was high and comparable in the three groups (table 2).
Table 2.
Echocardiographic (n = 179) and coronary angiographic (n = 99) features of patients with APO with STEMI, NSTEMI, and UA
| STEMI (a, n = 31) | NSTEMI (b, n = 109) | UA (c, n = 39) | p Value | |||
| a v b | a v c | b v c | ||||
| Ejection fraction (%) | 38.6 (12.0) | 40.7 (14.1) | 38.8 (13.1) | NS | NS | NS |
| <50% | 23 (74%) | 72 (66%) | 35 (90%) | NS | NS | NS |
| <40% | 17 (55%) | 49 (45%) | 20 (51%) | NS | NS | NS |
| Septum (mm) | 12.3 (2.9) | 12.5 (2.9) | 12.1 (2.8) | NS | NS | NS |
| Posterior wall (mm) | 12.0 (1.8) | 11.9 (2.6) | 11.7 (2.7) | NS | NS | NS |
| Moderate to severe MR | 5 (16%) | 37 (34%) | 13 (33%) | <0.011 | NS | NS |
| Abnormal LV relaxation | 13 (42%) | 52 (48%) | 15 (38%) | NS | NS | NS |
| Pseudonormalisation | 3 (10%) | 8 (7%) | 8 (20%) | NS | NS | NS |
| Restrictive pattern | 4 (13%) | 8 (7%) | 4 (10%) | NS | NS | NS |
| Coronary angiography | 21 (68%) | 55 (50%) | 23 (59%) | NS | NS | NS |
| Vessels with >70% stenosis | ||||||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) | NS | NS | NS |
| 1 | 3 (14%) | 3 (6%) | 3 (13%) | NS | NS | NS |
| 2 | 4 (19%) | 9 (16%) | 5 (22%) | NS | NS | NS |
| 3 ± left main | 14 (67%) | 43 (78%) | 15 (65%) | NS | NS | NS |
Data are mean (SD) or number (%).
LV, left ventricular; MR, mitral regurgitation.
Elderly patients with ACS with or without acute pulmonary oedema
Patients ⩾ 70 years old with acute pulmonary oedema had a higher incidence of old MI, systemic hypertension, diabetes mellitus, peripheral vascular disease, previous atrial fibrillation, and MI—particularly NSTEMI—than those without acute pulmonary oedema but the older patients had a smaller infarct size. They also had a lower ejection fraction and a higher rate of ventricular dilatation, moderate to severe MR, left ventricular diastolic dysfunction, and multivessel disease (table 3).
Table 3.
Demographic, echocardiographic, and angiographic data of patients with acute coronary syndromes aged ⩾70 years with or without APO
| APO (n = 75) | No APO (n:74) | p Value | |
| Age (years) | 75.1 (3.2) | 75.2 (3.1) | NS |
| Men | 44 (59%) | 49 (66%) | NS |
| Hypertension | 53 (69%) | 39 (53%) | <0.04 |
| Diabetes mellitus | 44 (59%) | 19 (26%) | <0.001 |
| Cholesterol >6.5 mmol/l | 26 (35%) | 35 (47%) | NS |
| PVD | 49 (65%) | 17 (23%) | <0.001 |
| Old myocardial infarction | 36 (48%) | 18 (24%) | <0.003 |
| Previous atrial fibrillation | 18 (24%) | 9 (12%) | <0.061 |
| Peak CKMB mass (μg/l) | 98 (165) | 161 (145) | <0.04 |
| Peak cTnI (μg/l) | 32.6 (74.7) | 57.6 (70.3) | <0.098 |
| STEMI | 16 (21%) | 27 (36%) | NS |
| NSTEMI | 45 (60%) | 22 (30%) | <0.001 |
| Left bundle branch block | 23 (31%) | 1 (1%) | <0.001 |
| UA | 14 (19%) | 25 (34%) | <0.03 |
| Ejection fraction (%) | 38.7 (13.0) | 56.1 (10.2) | <0.001 |
| Septum (mm) | 12.3 (2.8) | 12.0 (2.1) | NS |
| Posterior wall (mm) | 11.7 (1.9) | 11.2 (2.1) | NS |
| Moderate to severe MR | 27 (36%) | 7 (10%) | <0.001 |
| Coronary angiography | 46 (61%) | 31 (42%) | NS |
| Vessels with >70% stenosis | |||
| 0 | 0 (0%) | 0 (0%) | NS |
| 1 | 3 (7%) | 12 (39%) | NS |
| 2 | 7 (15%) | 8 (26%) | NS |
| 3 ± left main | 36 (78%) | 11 (35%) | <0.001 |
Data are mean (SD) or number (%).
In-hospital events
The incidence of systemic hypotension (systolic blood pressure < 100 mm Hg) during the first 24 hours was higher in patients with STEMI (38% v 18% in NSTEMI and 18% in UA, p < 0.04). Patients with STEMI also had a higher need for endotracheal intubation and mechanical ventilation (21% v 13% and 5%, respectively, p < 0.05) but a similar recurrence of acute pulmonary oedema (9% v 13% and 13%, respectively, not significant). Thrombolytic was given to three patients, all with STEMI, and primary angioplasty was performed in six with STEMI and in three with NSTEMI. Coronary revascularisation was performed in 38 patients (20%): percutaneous in 21 (11%) and surgical in 17 (9%). Of the remaining 61 patients who also underwent an angiography, revascularisation procedures were not carried out because 46 had unsuitable vessels, five died early, eight had major co-morbidity factors, and two patients declined the procedures.
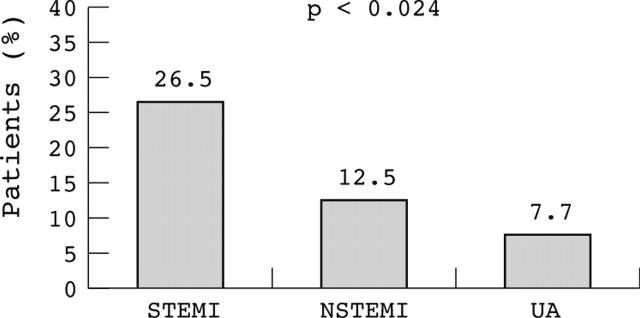
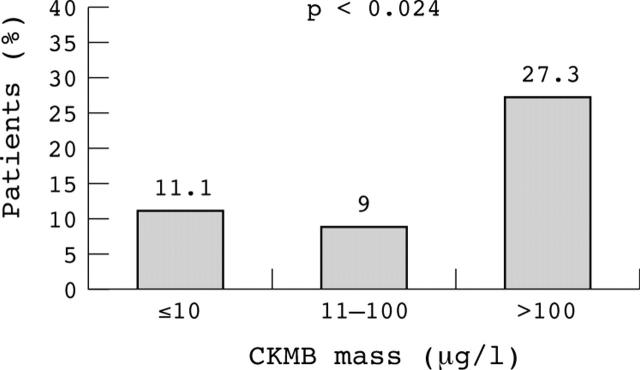
Twenty six patients died during the first 30 days (14.1%), 20 of cardiogenic shock and six of sepsis or respiratory distress. Global mortality was higher in patients with STEMI than in those with NSTEMI (fig 1) and was highest among those with a large MI (fig 2). Cardiac mortality was also higher in patients with STEMI (10 of 39 (26%) v 10 of 112 (9%) in NSTEMI and 0 of 34 (0%) in UA, p < 0.024). Moreover, mortality was higher among patients with systemic hypotension on admission (systolic blood pressure < 100 mm Hg in five of 26 (19.2%) v ⩾ 100 mm Hg in seven of 159 (4.4%), p < 0.001), need for intubation (12 of 26 (46.2%) v 12 of 159 (7.5%), p < 0.001), recurrence of acute pulmonary oedema (nine of 26 (34.6%) v 17 of 159 (11%), p < 0.001), or a reduced ejection fraction (< 40% in 18 of 86 (20.9%) v ⩾ 40% in five of 93 (5.4%), p < 0.001). A multivariate regression analysis identified a reduced ejection fraction (< 40%, p < 0.027) and a peak CKMB concentration > 100 μg/l (p < 0.015) as the main independent prognostic markers. Although mortality in revascularised patients tended to be lower than in non-revascularised patients (three of 35 (8.6%) v 23 of 150 (15.3%), p = 0.272), non-revascularised patients tended to be more seriously ill.
Figure 1.
Thirty day mortality among patients with acute pulmonary oedema was higher in those with ST segment elevation myocardial infarction (STEMI) than in those with non-ST segment elevation myocardial infarction (NSTEMI) or with unstable angina (UA).
Figure 2.
Relation between peak creatine kinase MB (CKMB) mass and 30 day mortality among patients with acute coronary syndromes complicated with acute pulmonary oedema. Mortality was higher in patients with larger enzymatically estimated infarct size.
DISCUSSION
Our study showed that NSTEMI and UA were the most frequent ACS underlying acute pulmonary oedema (82%) but that mortality was highest among patients with STEMI and was mostly related to extensive necrosis. It also documented that most patients in the three subsets had systolic and diastolic dysfunction and a high incidence of multivessel disease.
STEMI, NSTEMI, and UA
Patients with STEMI had a lower incidence of hypertension, diabetes, previous MI, atrial fibrillation, or previous admission for heart failure, but a larger infarct size than those with NSTEMI or UA. Patients with NSTEMI or UA, however, had a comparable clinical profile. The few existing studies on acute pulmonary oedema associated with ACS have not analysed either the frequency of these different presentations or the possible differences in their clinical, ECG, echocardiographic, or angiographic features.1,4,5,6,7,8,9,10,11 Warnowicz et al,11 for example, analysed 39 patients with MI and acute pulmonary oedema but did report the kind of ECG changes or compare the results with patients with UA. Similarly, Clark et al5 evaluated 56 patients with coronary disease without MI but did not analyse the ECG, clinical, and angiographic features. Goldberger et al,1 in a series of 94 patients with acute pulmonary oedema, distinguished 45 patients with ACS but did not report demographic or angiographic data. In another study, Wiener et al9 noted that, among 44 patients with coronary disease and acute pulmonary oedema, the 26 with MI had a higher mortality (46%) than the rest (6%) but they reported no data on enzymatic peak, ventricular function, or coronary anatomy, or the possible differences between STEMI and NSTEMI patients.9 More recently, Edoute et al10 identified clinically 128 patients with coronary artery disease and acute pulmonary oedema and distinguished those with stable angina (37%), UA (30%), Q wave MI (13%), and non-Q wave MI (6%), but they also did not analyse possible differences between these groups and reported no angiographic data.
Left ventricular function and MR
The incidence of left ventricular systolic dysfunction was high and comparable in our three groups. However, the acute deterioration was probably more remarkable among patients with STEMI than among patients with NSTEMI or UA because patients with STEMI had a significantly lower rate of previous MI and a larger infarct size. Left ventricular diastolic dysfunction, on the other hand, was also highly prevalent in the three groups, although it was more severely impaired in patients with NSTEMI or UA, possibly because these two groups had a higher incidence of previous MI, chronic systemic hypertension,12 and diabetes.13 In the three groups, however, additional diastolic dysfunction was caused by the acute ischaemic insult and the corresponding further reduction in left ventricular compliance.14,15 In this respect, existence of a severe underlying coronary disease with a strikingly high incidence of three vessel and left main disease might have lowered the ischaemic threshold in areas distal to the territory of the culprit lesion during the admitting episode. They might also have experienced ischaemia as a result of a multiple plaque reactivity.16–18
The three forms of ACS also shared the frequent presence of MR, which, in all likelihood, had also contributed to acute pulmonary oedema, particularly in patients with NSTEMI or UA. This is a relevant finding because the severity of MR at the time of acute pulmonary oedema was probably greater than that measured, since MR was not generally assessed in the very acute phase when the ischaemic episode was associated with intense peripheral vasoconstriction.
Clinical outcome
In our series, mortality was unrelated to age and was highest among patients with STEMI (26.5%), especially in those with large myocardial necrosis, and lowest among patients with UA (7.7%). These results are consistent with the results of Golberger et al,1 who found that in-hospital mortality in 45 patients with acute pulmonary oedema was also lower in patients with UA (5.6%) than in patients with STEMI (18.2%). More recently, Rott et al4 reported a 35% mortality rate at 30 days in 150 patients in Killip class III, including both STEMI and NSTEMI, identical to the findings in the original series from Killip and Kimball19 and comparable with other contemporary studies of patients with STEMI where 30 day mortality ranged from 27–36%.2,3,20 However, our study provides additional information about the prognostic relevance of a peak CKMB concentration > 100 μg/l, the presence of systemic hypotension on admission—also pointed out by others1,21—and a reduced ejection fraction (< 40%). These results, therefore, underscore the greater prognostic role of the extent and abruptness of the systolic loss rather than the severity of diastolic dysfunction in the 30 day mortality. We also observed that mortality was lower in revascularised than in non-revascularised patients and this may reflect a net benefit from revascularisation. Nevertheless, ours was not a randomised intervention and, in fact, non-revascularised patients tended to be more seriously ill than those who were revascularised.
Advanced age, ACS, and acute pulmonary oedema
Advanced age has been a recognised feature of patients with acute pulmonary oedema.1,9,10 In the present study, however, we analysed the contribution of factors other than advanced age by comparing patients with ACS aged ⩾ 70 years with acute pulmonary oedema with those without acute pulmonary oedema. This comparison further emphasised the role of hypertension, diabetes, low ejection fraction, MR, and multivessel disease as remarkable contributors to acute pulmonary oedema.
Limitations
Although coronary angiography documented extensive disease in most of our patients, this procedure could not be performed in all of them, thereby limiting the consistency of the observation of the extent of disease. In general, however, angiography was less often performed in more elderly patients and in patients with more adverse co-morbidity. Hence, we think it unlikely that the subset left out of angiography would have had less extensive coronary disease than those with angiography. In fact, in most instances the medical decision to withhold coronary revascularisation was the main limiting factor to angiography rather than the co-morbidity. Moreover, other investigators have also found extensive coronary disease in patients with acute pulmonary oedema, although in isolated cases or in much smaller series.5,9,11,22 Also, evaluation of diastolic function was hampered by the lack of assessment of pulmonary veins. Another possible drawback, the rather reduced number of patients with STEMI, seems to reflect the small proportion of acute pulmonary oedema among these patients2–4,20 because, at least in our centre, patients with STEMI still outnumber those with NSTEMI. Finally, in patients with small increases in biochemical markers of necrosis it is unclear whether these increased markers contributed to causing acute pulmonary oedema or whether they were the consequence of increased left ventricular wall tension, particularly in view of the reduced number of early angiographies.
Implications
Irrespective of the kind of underlying ACS, patients with acute pulmonary oedema have several common clinical characteristics such as a high incidence of chronic hypertension, diabetes, and previous MI, which favour diastolic and systolic dysfunction. They also had a high incidence of multivessel disease and MR, which underscores the appropriateness of vasodilating agents to reduce afterload and to improve coronary blood flow, as well as the need to perform a coronary angiography whenever allowed by co-morbidity factors. Nevertheless, since most patients with acute pulmonary oedema have a minor NSTEMI or UA it seems more pertinent to have their catheterisation procedures deferred until haemodynamic and ventilatory functions stabilise. In contrast, in patients with STEMI, and mainly due to the larger extent of their necrosis, it appears to be more advantageous to perform urgent catheterisation and revascularisation aimed at reducing infarct size and subsequent mortality.
Abbreviations
ACS, acute coronary syndromes
CK, creatine kinase
LBBB, left bundle branch block
MI, myocardial infarction
MR, mitral regurgitation
NSTEMI, non-ST segment elevation myocardial infarction
STEMI, ST segment elevation myocardial infarction
UA, unstable angina
REFERENCES
- 1.Goldberger JJ, Peled HB, Stroh JA, et al. Prognostic factors in acute pulmonary edema. Arch Intern Med 1986;146:489–93. [PubMed] [Google Scholar]
- 2.GISSI Investigators. Long-term effects of intravenous thrombolysis in acute myocardial infarction: final report of the GISSI study. Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico (GISSI). Lancet 1987; ii :871–4. [PubMed]
- 3.Lee KL, Woodlief LH, Topol EJ, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction: results from an international trial of 41,021 patients. Circulation 1995;91:1659–68. [DOI] [PubMed] [Google Scholar]
- 4.Rott D, Behar S, Gottlieb S, SPRINT Study Group, et al. Usefulness of the Killip classification for early risk stratification of patients with acute myocardial infarction in the 1990s compared with those treated in the 1980s. Am J Cardiol 1997;80:859–64. [DOI] [PubMed] [Google Scholar]
- 5.Clark LT, Garfein OB, Dwyer EM Jr. Acute pulmonary edema due to ischemic heart disease without accompanying myocardial infarction: natural history and clinical profile. Am J Med 1983;75:332–6. [DOI] [PubMed] [Google Scholar]
- 6.Kunis R, Greenberg H, Yeoh CB, et al. Coronary revascularization for recurrent pulmonary edema in elderly patients with ischemic heart disease and preserved ventricular function. N Engl J Med 1985;313:1207–10. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi H, Iwasaka T, Sugiura T, et al. Acute pulmonary edema in patients with unstable angina: clinical profile and natural history. Coron Artery Dis 1992;3:529–32. [Google Scholar]
- 8.Sharkey SW, Aberg NB. Hemodynamic evidence of painless myocardial ischemia with acute pulmonary in coronary disease. Am Heart J 1995;129:188–91. [DOI] [PubMed] [Google Scholar]
- 9.Wiener RS, Moses HW, Richeson JF, et al. Hospital and long-term survival of patients with acute pulmonary edema associated with coronary artery disease. Am J Cardiol 1987;60:33–5. [DOI] [PubMed] [Google Scholar]
- 10.Edoute Y, Roguin A, Behar C, et al. Prospective evaluation of pulmonary edema. Crit Care Med 2000;28:330–5. [DOI] [PubMed] [Google Scholar]
- 11.Warnowicz MA, Parker H, Cheitlin MD. Prognosis of patients with acute pulmonary edema and normal ejection fraction after acute myocardial infarction. Circulation 1983;67:330–4. [DOI] [PubMed] [Google Scholar]
- 12.Topol EJ, Traill TA, Fortuin NJ. Hypertensive hypertrophic cardiomyopathy of the elderly. N Engl J Med 1985;312:277–83. [DOI] [PubMed] [Google Scholar]
- 13.Stone PH, Muller JE, Hartwell T, et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. J Am Coll Cardiol 1989;14:49–57. [DOI] [PubMed] [Google Scholar]
- 14.Parker JO. Hemodynamic and metabolic changes during myocardial ischemia. Arch Intern Med 1972;129:947–61. [PubMed] [Google Scholar]
- 15.Figueras J, Singh BN, Ganz W, et al. Mechanisms of rest and nocturnal angina: observations during continuous hemodynamic and electrocardiographic monitoring. Circulation 1979;59:955–68. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein JA, Demetrriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med 2000;343:915–22. [DOI] [PubMed] [Google Scholar]
- 17.Rioufol G, Finet G, Ginon I, et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation 2002;106:804–8. [DOI] [PubMed] [Google Scholar]
- 18.Figueras J, Juncal A, Cortadellas J, et al. Relevance of multivessel disease in the development of in-hospital refractory angina and myocardial infarction in patients with unstable angina. Int J Cardiol 2004;94:221–7. [DOI] [PubMed] [Google Scholar]
- 19.Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit: a two-year experience with 250 patients. Am J Cardiol 1967;20:457–64. [DOI] [PubMed] [Google Scholar]
- 20.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial, Lancet 1999;354:716–22. [DOI] [PubMed] [Google Scholar]
- 21.Fedullo AJ, Swinburne AJ, Wahl GW, et al. Acute cardiogenic pulmonary edema treated with mechanical ventilation: factors determining in-hospital mortality. Chest 1991;99:1220–6. [DOI] [PubMed] [Google Scholar]
- 22.Graham SP, Vetrovec GW. Comparison of angiographic findings and demographic variables in patients with coronary artery disease presenting with acute pulmonary edema versus those presenting with chest pain. Am J Cardiol 1991;68:1614–8. [DOI] [PubMed] [Google Scholar]