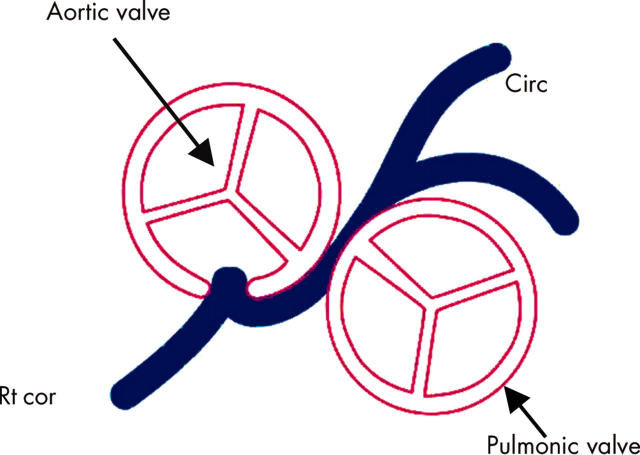
Normal coronary artery anatomy is characterised by two ostia centrally placed in the right and left sinus of Valsalva. The main left coronary artery (LCA) originates from the left ostium, branching into the left anterior descending artery and circumflex artery, which courses around the left atrioventricular groove; the right coronary artery (RCA) arises from the right ostium, providing an infundibular branch to the anterior side of the heart, and then courses backward in the atrioventricular groove. The three main coronary arteries branch superiorly to the atria and inferiorly to the ventricles; they end in broom-like arborisations, which penetrate the myocardium.1 Because of ventricular contraction myocardial perfusion of the left ventricle occurs mainly in diastole, while the myocardium of the right ventricle is perfused during both heart cycles.2–4
Clinical suspicion that a patient’s problems may be the result of coronary anomalies remains an important challenge in diagnosis, especially in children. Haemodynamically significant congenital anomalies of coronary arteries occur as isolated or primary forms and as secondary forms in association with congenital heart disease (CHD) (pulmonary atresia with intact interventricular septum or hypoplastic left heart syndrome with aortic atresia and severe mitral stenosis) (table 1). In this article only isolated/primary forms will be discussed.
Table 1.
Haemodynamically significant congenital anomalies of the coronary arteries
| Isolated/primary—without CHD |
| • Anomalous origin of accessory coronary arteries from the pulmonary artery (ALCAPA, ARCAPA) |
| • Ectopic origin of the coronary arteries from aortic sinus |
| • Absence of a coronary artery |
| • Congenital coronary artery fistula |
| Secondary—with CHD |
| • PA + IVS |
| • AA + MS |
AA, aortic atresia; ALCAPA, anomalous origin of the left coronary artery from the pulmonary artery; ARCAPA, anomalous origin of the right coronary artery from the pulmonary artery; CHD, congenital heart disease; IVS, interventricular septum; MS, mitral stenosis; PA, pulmonary atresia.
INCIDENCE
Overall, anomalies of the coronary arteries are rather rare and the incidence of primary congenital coronary anomalies varies from 0.3% in a necropsy series reported by Alexander and Griffith to 1.6% of patients undergoing cardiac catheterisation in a series of more than 38 000 patients.5 Although these anomalies are rare, they may be seen with haemodynamic or myocardial perfusion abnormalities or high risk anatomy for accelerated atherosclerosis; they may result in symptoms varying from dyspnoea to sudden death. The milder forms escape detection both during life and at postmortem examination.
CLASSIFICATION
The classification of primary forms of coronary artery anomalies varies between ectopic origin of the coronary arteries from the aortic sinus, stenosis of the coronary arteries, absence of a coronary artery, to anomalous origin of the left or right coronary artery from the pulmonary artery or congenital coronary artery fistula.
SYMPTOMS
Although the spectrum of symptoms and associated syndromes is broad, the coronary artery anomalies that give rise to symptoms are limited to those that cause significant alteration in myocardial perfusion or result in pronounced left-to-right shunting (steal). The coronary anomalies most likely to cause myocardial infarction, ischaemia, or ventricular tachycardia are anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), large coronary arteriovenous fistulas, and those anomalies associated with a coronary artery coursing between the great vessels. The other coronary artery anomalies are rarely associated with symptoms or sudden death.
ANOMALOUS ORIGIN OF THE LEFT CORONARY ARTERY
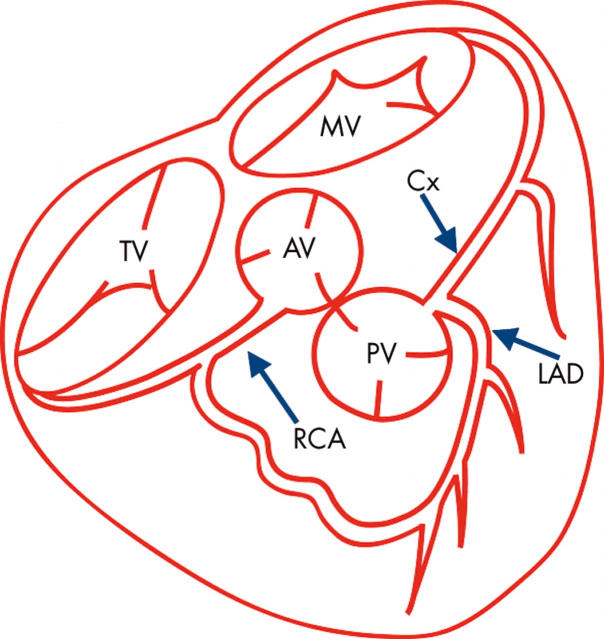
Anomalous origin of the left main coronary artery (LMCA) (fig 1), the left anterior descending or circumflex branch from the proximal pulmonary artery or more distally to the proximal right pulmonary artery is a rare congenital anomaly occurring in 0.26% of patients with congenital heart disease undergoing cardiac catheterisation.6 In 1933 Bland, White and Garland described the clinical syndrome associated with this anomaly based on their experience with a 3 month old infant who died from it.7
Figure 1.
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA). AV, aortic valve; Cx, circumflex coronary artery; LAD, left anterior descending coronary artery; MV, mitral valve; PV, pulmonic valve; RCA, right coronary artery; TV, tricuspid valve.
When the RCA originates from the pulmonary trunk (ARCAPA), an anomaly rarer than ALCAPA, there is no ischaemia because the right ventricle is under low pressure; without ischaemia there is no stimulus to form collateral anastomoses with branches of the LCA and thus there is no great tendency to establish fistulous flow.
Both coronary arteries arising from the pulmonary trunk constitute an extremely rare situation. In such cases symptoms appear within a few days of birth, and death follows within two weeks; it is compatible with life only if associated with pulmonary hypertension.
Since Edwards’ fundamental contribution,8 it has been known that dramatic changes occur in the coronary circulation in all children with ALCAPA within the first weeks of life. While the fetus remains in utero, the heart develops quite normally. After birth, as long as the pulmonary arterial pressure remains at or near systemic levels, the left ventricular myocardium supplied by the anomalous artery remains well perfused; the avidity of myocardium for oxygen is such that normal perfusion with blood of mixed venous oxygen content does not present a problem.
As pressure in the pulmonary trunk falls postnatally, perfusion of the left ventricle begins to suffer because the period during which pressure in the coronary artery exceeds intramural left ventricular pressure becomes shorter. The consequence of the circulatory handicap is a decrease of left ventricular function and an increase of left ventricular end diastolic pressure with consecutive pulmonary vasoconstriction; slowing of the postnatal reduction of pulmonary artery pressure and migration of the perfusion problem will result. At the same time, the ischaemic myocardium is being perfused increasingly by a developing set of collateral vessels from the RCA which arise normally.
Three groups of patients can be identified:
those with perfectly distributed collateral arteries before the pulmonary artery pressure had fallen with minor ischaemic damage of the left ventricular myocardium, which is well perfused, and normal in form and function; this type is often diagnosed in adolescents or adulthood
those with poorly distributed collateral connections—some parts of the left ventricle are well perfused while others become ischaemic
those with collateral maldistribution, where there are large interconnections proximal to the branches supplying contracting myocardium which act as a left-to-right arterial shunt, requiring extra work for the left ventricle while deviating blood supply from the myocardium (steal from the myocardium to the pulmonary artery).
Thus this malformation postnatally presents a picture which may vary enormously from case to case and from time to time in a given patient (table 2).
Table 2.
Symptomatology of ALCAPA
| • Perfectly distributed collateral arteries: minor ischaemic damage |
| • Collaterals poorly distributed: some parts well perfused/some ischaemic |
| • Large interconnections: steal |
The small infant usually presents with signs of congestive heart failure and a particular type of anginal attack, while the older child and adolescent present with an unexplained heart murmur caused by mitral insufficiency, mild cardiomegaly, or an abnormal ECG. Myocardial ischaemia may present first in adolescence or young adult life when, under stress or maximal motivated exertion, either anginal pain or arrhythmia may occur. The latter may result in sudden unexplained death or near death. While the ECG is not likely to be normal, it may not show the classical findings in ischaemia or infarction, so that there are occasions with this disease when the ECG is not diagnostic. The older child who has survived without symptoms may very well have a normal ECG.
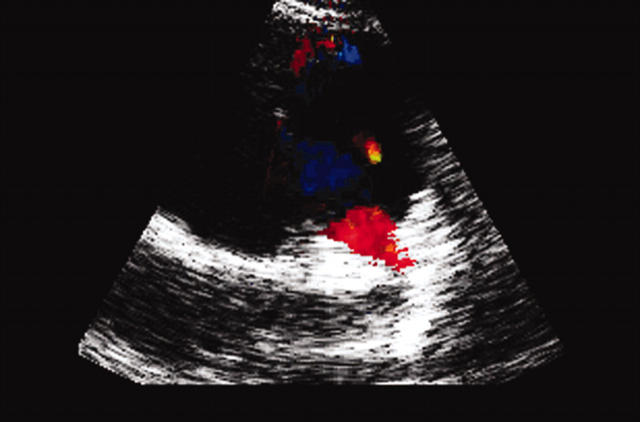
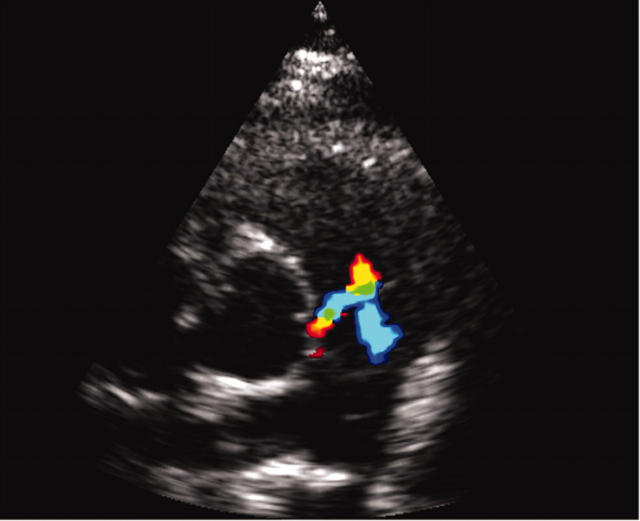
All echocardiographic screening studies should attempt two dimensional imaging of the origin of the two coronary arteries; however, definite diagnosis requires visualisation of the anomalous origin. The inability to find the orifice of a coronary artery is no proof of its absence or of its anomalous origin elsewhere. Abnormal flow is seen in ALCAPA on echocardiography, where diastolic retrograde flow from the LMCA to the pulmonary artery is initially identified by colour flow imaging and then confirmed with pulse wave Doppler (fig 2).
Figure 2.
Echocardiogram of a patient with ALCAPA showing abnormal flow.
Another finding associated with ALCAPA is a dilated RCA (fig 3) caused by increased flow, left ventricular enlargement, and depressed ventricular function.
Figure 3.
Dilated right coronary artery associated with ALCAPA.
Cardiac catheterisation remains the gold standard in the diagnosis of coronary artery anomalies. The objective of angiocardiography is to establish the integrity and source of the coronary arteries. An aortic root injection may be sufficient to show the early filling of the RCA and the delayed passage via collateral vessels into the LCA and finally the pulmonary trunk.
The finding of coronary artery filling from the pulmonary trunk should rule out ligation of the LMCA at its origin as a form of treatment for the patient.
The diagnosis of ALCAPA in an infant, usually seriously ill, is an indication for urgent surgery; diagnosis in older patients should also be an indication for surgery. Excellent surgical results have been reported following re-establishment of a dual coronary artery system, with direct reimplantation of the coronary artery into the aorta,9 left subclavian artery to LCA anastomosis, or transpulmonary baffling or Takeuchi procedure,10 as it restores oxygenated blood flow to the left ventricular myocardium immediately and more completely. Although clinical improvement with simple ligature of the LCA is possible in individual cases, perioperative mortality associated with this operation seems higher and late results of left ventricular function are less satisfactory.11
Generally, in infants and young children, dramatic improvement of left ventricular function occurs within the first year after establishment of a dual coronary artery system. The younger the patient the quicker the recovery of ventricular function; this probably reflects the enormous potential for tissue growth in infants which is an argument for operating early. Global left ventricular function was found to be reduced only when major structural anomalies such as left ventricular aneurysm, obstruction of the anastomosis of the LCA with the aorta, and mitral valve replacement were present.
Children with myocardial scars on postoperative nuclear imaging generally have undergone surgery at an older age. This indicates that myocardial ischaemia in ALCAPA may result in more pronounced scarring when surgery is done later in life, emphasising the importance of early surgical intervention as soon as the diagnosis is established.12
Positron emission tomography, as a non-invasive method for quantitative assessment of myocardial perfusion, can show that myocardial blood flow and coronary flow reserve are greatly reduced in the LCA territories of long term survivors of ALCAPA repair, irrespective of the angiographic status of the graft. This may result in myocardial ischaemia during periods of increased oxygen demand such as exercise, contributing to impaired exercise performance.
Although the extent and severity of stress perfusion defects in adults with coronary artery disease have been shown to have prognostic significance and relate to adverse cardiovascular events, it is unknown whether these prognostic indicators are applicable to long term survivors of ALCAPA repair.
Myocardial perfusion imaging or stress echocardiography should be used to identify patients with severely reduced flow reserve and exercise induced ischaemia. Advice on physical activity should be based on the result of these imaging studies. Anti-ischaemic medications (β blockers) should be considered in patients with evidence of exercise induced myocardial ischaemia or severely reduced myocardial flow reserve. In addition, patient education about known risk factors for coronary artery disease is important.
MINOR CORONARY VARIATIONS
Abnormalities of the origin of the coronary arteries from the aorta are referred to as minor coronary variations. Most of these anomalies are of little clinical importance during the paediatric years. Clinical events are related to this condition only when the anomalously connecting artery passes between the aorta and the pulmonary trunk.13 They may become important in adolescence and young adult life when extremes of physical exertion may cause significant intolerance of the previously well tolerated malfunction. These abnormalities may also be significant in middle age when the additional effects of atherosclerotic changes have become clinically manifest.
Origin of the left main coronary artery from the right sinus of Valsalva
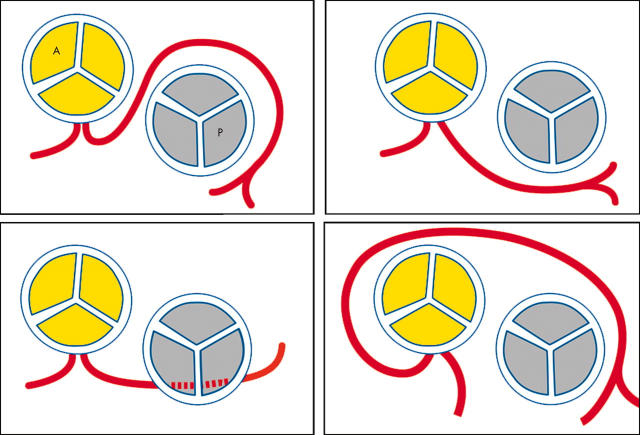
The origin of the LMCA from the right sinus of Valsalva can be classified into four types (fig 4):
Figure 4.
Origin of the left main coronary artery (LMCA) from the right sinus of Valsalva. Top left: the LMCA passes between the aorta and pulmonary trunk, posteriorly and adjacent to the pulmonary trunk. Top right: the LMCA passes anteriorly over the right ventricular outflow tract. Bottom left: the LMCA may course along the crista supraventricularis intramyocardially or subendocardially, surfacing in the proximal interventricular sulcus. Bottom right: the LMCA may rise to the right of the right coronary artery and pass posteriorly to the aortic root.
the LMCA passes between the aorta and pulmonary trunk, posteriorly and adjacent to the pulmonary trunk
the LMCA passes anteriorly over the right ventricular outflow tract
the LMCA may course along the crista supraventricularis intramyocardially or subendocardially, surfacing in the proximal interventricular sulcus
the LMCA may rise to the right of the RCA and pass posteriorly to the aortic root.
Cheitlin and his colleagues14 have pointed out the pathologic significance of a single coronary artery or of both coronary arteries from the right sinus of Valsalva when the branch or artery that supplies the left coronary distribution courses leftward between the aorta and pulmonary artery so that it can be compressed (fig 5). They showed a striking incidence of sudden unexplained death in adolescents and young adults, and a strong relation of death to heavy exertion. For this reason, any child or young person with angina pectoris, myocardial infarction, or cardiac syncope should have this anomaly ruled out by coronary angiography.
Figure 5.
Coronary artery compression.
In contrast, when a single coronary artery arises from the left sinus of Valsalva or both coronaries arise from separate ostia in that sinus with the RCA supply coursing between aorta and right ventricular outflow tract, compression can occur but sudden death usually does not, probably because of the low perfusion pressure required for the right ventricle.
An interesting finding was published by Bellhouse and colleagues15 in 1968: they reported that the coronary blood flow is significantly attenuated if the orifice of the coronary arteries lies above the sinus of Valsalva beyond the sinotubular junction. This finding should be taken into consideration in patients with transposition of the great arteries after the arterial switch operation with reimplantation of the coronary arteries into the ascending aorta.
Indications for surgery remain debatable; however, reversible ischaemia in the anomalously connecting artery is an indication. Coronary artery bypass grafting, using the internal thoracic artery graft to the anterior descending artery and a saphenous vein graft to the circumflex artery, is feasible in patients with anomalous connection of the LMCA to the right sinus of Valsalva. Alternatively, the intramural part of the coronary artery can be opened longitudinally from within the aorta, with reconstruction of the most proximal part of the coronary artery, so that the ostium emerges from the left sinus of Valsalva in a normal and unobstructed location.16 The absence of the LMCA was noted as the most common abnormality involving the coronary arterial system, with an angiographic incidence of 0.4–0.5% of all coronary arteriograms.
Congenital arteriovenous/arterio-arterial fistulae
Congenital arterio-venous or arterio-arterial fistulae (CAF) of the coronary arteries are direct connections between a branch of a coronary artery and the lumen of the four cardiac chambers, the coronary sinus, the superior vena cava, the pulmonary artery, or the pulmonary veins close to the heart without the intervening capillary system. The source is most often the RCA with the LCA being much less involved.17 Embryologically, these fistulae seem to represent persistent junctions of primordial epicardial vessels with intramyocardial sinusoidal circulation. Most patients present late in life, occasionally in childhood and rarely in infancy.
Symptoms of the disease may be a continuous systolic–diastolic murmur, congestive heart failure, pulmonary hypertension, bacterial endocarditis in 5% of cases,9 premature arteriosclerotic changes within the fistulae, and thromboembolic events. Angina is uncommon and myocardial infarction rare; it is postulated that these ischaemic symptoms are caused by coronary steal.18
CAF that have not been detected or closed in childhood have been reported to become symptomatic in adulthood because of chronic volume load and ischaemia. Thus, it has previously been recommended that these fistulae be closed in childhood, either surgically or, as suggested more recently, by transcatheter coil occlusion. Incidental detection of CAF in asymptomatic patients has been noted on coronary angiography, but their clinical significance is unclear.
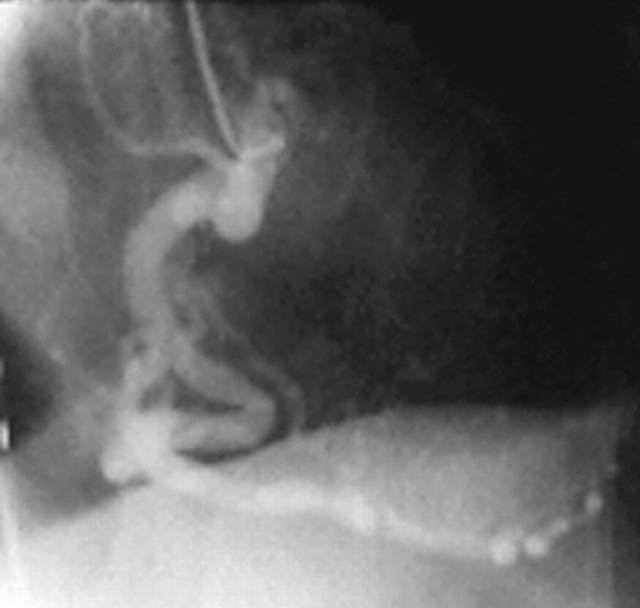
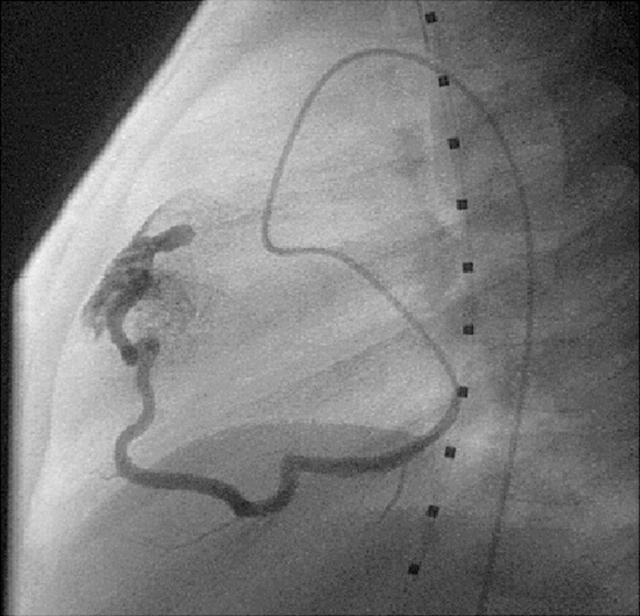
With the advent of high resolution two dimensional and colour Doppler echocardiography during the past decade, the incidental diagnosis of clinically silent CAF is increasing. Colour flow mapping has proved to be a highly sensitive method for identifying small CAF (fig 6). Cardiac catheterisation and selective coronary angiography are necessary for definitive diagnosis and planning of either surgical repair or occlusion by interventional catheterisation19 (fig 7).
Figure 6.
Colour Doppler echocardiography used for detecting congenital arterio-venous or arterio-arterial fistulae (CAF).
Figure 7.
Coronary angiogram during interventional catheterisation in a patient with CAF.
Currently, there is a paucity of information regarding the clinical implications of incidentally identified, clinically silent CAF. Some authors have recommended elective closure of CAF regardless of symptoms, while others have advocated conservative management. The current general opinion does not support the previous recommendations to close CAF even in asymptomatic patients; a compelling argument in favour of a conservative approach to these patients is the unexpectedly high incidence of spontaneous closure of CAF.
Several clinically relevant questions remain unanswered. The life long risk of complications such as infective endocarditis, aneurysm formation, dissection, rupture, accelerated atherosclerosis, and thromboembolism is not known. Nevertheless, because any intervention to close such fistulae is associated with a risk of morbidity, and because the benefit of such intervention is questionable, a conservative approach to small asymptomatic CAF is recommended.
MULTIPLE CHOICE QUESTIONS.
Education in Heart Interactive (www.heartjnl.com/misc/education.shtml)
There are six multiple choice questions associated with each Education in Heart article (these questions have been written by the authors of the articles). Each article is submitted to EBAC (European Board for Accreditation in Cardiology; www.ebac-cme.org) for 1 hour of external CPD credit.
How to find the MCQs: Click on the Online Learning: [Take interactive course] link on the table of contents for the issue online or on the Education in Heart collection (www.heartjnl.com/cgi/collection/heart_education).
Free access: This link will take you to the BMJ Publishing Group’s online learning website. Your Heart Online user name and password will be recognised by this website. As a Heart subscriber you have free access to these MCQs but you must register on the site so you can track your learning activity and receive credit for completed courses.
How to get access: If you have not yet activated your Heart Online access, please do so by visiting http://www.bmjjournals.com/cgi/activate/basic and entering your six digit (all numeric) customer number (found above your address label with your print copy). If you have any trouble activating or using the site please contact subscriptions@bmjgroup.com
Case based Heart: You might also be interested in the interactive cases published in association with Heart (http://cpd.bmjjournals.com/cgi/hierarchy/cpd_node;CBH)
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
REFERENCES
- 1.Gregg DE, Fisher LC. Blood supply to the heart. In: Handbook of physiology. Washington DC: American Physiological Society, 1963.
- 2.Mosher P, Ross J Jr, McFate PA, et al. Control of coronary blood flow by an autoregulatory mechanism. Circ Res 1964;14:250–9. [DOI] [PubMed] [Google Scholar]
- 3.Epstein SE, Cannon RO, Talbot TL. Hemodynamic principles in the control of coronary blood flow. Am J Cardiol 1985;56:4–10. [DOI] [PubMed] [Google Scholar]
- 4.Fulton WFM. Morphology of the myocardial microcirculation. Br Heart J 1964;26:39–50.14106123 [Google Scholar]
- 5.Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation 1956;14:800–5. [DOI] [PubMed] [Google Scholar]
- 6.Askenazi J, Nadas AS. Anomalous left coronary artery originating from the pulmonary artery. Report on 15 cases. Circulation 1975;51:976. [DOI] [PubMed] [Google Scholar]
- 7.Bland EF, White PD, Garlang J. Congenital anomalies of the coronary arteries: report of an unusual case associated with cardiac hypertrophy. Am Heart J 1933;8:787. [Google Scholar]
- 8.Edwards JE. Functional pathology of congenital cardiac disease. Pediatr Clin North Am 1954;1:13. [DOI] [PubMed] [Google Scholar]
- 9.Kirklin JW, Barrat-Boyes BG. Cardiac surgery. volume II, 3 ed. Churchill Livingstone 2003 1251.
- 10.Takeuchi S, Imamura H, Katsumoto J, et al. New surgical method for repair of anomalous left coronary artery from the pulmonary artery. J Thorac Cardiovasc Surg 1979;78:7. [PubMed] [Google Scholar]
- 11.Kreutzer C, Schlichter AJ, Roman MI, et al. Emergency ligation of anomalous left coronary artery arising from the pulmonary artery. Ann Thorac Surg 2000;69:1591. [DOI] [PubMed] [Google Scholar]
- 12.Alexanderson E, Attie F, Zabal C, et al. Surgical treatment of anomalous origin of the left coronary artery from the pulmonary artery: late results evaluated with dobutamine stress gated single-photon emission computed tomography sestamibi study. J Nucl Cardiol 1997;4:341–2. [DOI] [PubMed] [Google Scholar]
- 13.Kragel AH, Roberts WC. Anomalous origin of either the right or left main coronary artery from the aorta with subsequent coursing between aorta and pulmonary trunk: analysis of 32 necropsy cases. Am J Cardiol 1988;62:771. [DOI] [PubMed] [Google Scholar]
- 14.Cheitlin MD, DeCastro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva: a not so minor congenital anomaly. Circulation 1974;50:780–7. [DOI] [PubMed] [Google Scholar]
- 15.Bellhouse BJ, Bellhouse FH. Fluid mechanics of the aortic coronary flow. Nature 1968;219:1059–61. [DOI] [PubMed] [Google Scholar]
- 16.Mustafa I, Gula G, Ridley-Smith R, et al. Anomalous origin of the left coronary artery from the anterior aortic sinus: a potential cause of sudden death. Anatomic characterization and surgical treatment. J Thorac Cardiovasc Surg 1981;82:297. [PubMed] [Google Scholar]
- 17.Liberthson RR, Sagar K, Berkoben JP, et al. Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation 1997;59:849. [DOI] [PubMed] [Google Scholar]
- 18.Rittenhouse EA, Doty DB, Ehrenhaft JL. Congenital coronary artery chamber fistula. Ann Thorac Surg 1975;20:468. [DOI] [PubMed] [Google Scholar]
- 19.Sediq M, Wilkinson JL, Qureshi SA. Successful occlusion of a coronary arteriovenous fistula using an Amplatzer duct occluder. Cardiol Young 2001;11:84. [DOI] [PubMed] [Google Scholar]